
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.14, Problem 30P
What
a.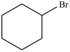 b.
b. 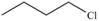 c.
c. 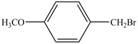
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In general, which is more polar, the stationary phase or the mobile phase?
The stationary phase is always more polar
The mobile phase is always more polar
It depends on our choices for both stationary and mobile phase
Their polarity doesn't really matter so we never consider it
Please help
Draw the mechanism of aspirin synthesis in an basic medium and in a neutral medium, showing the attacks and the process for the formation of the product.
Chapter 17 Solutions
Organic Chemistry (6th Edition)
Ch. 17.1 - Prob. 1PCh. 17.2 - Which carbonyl groups in the anticancer drug taxol...Ch. 17.2 - Prob. 3PCh. 17.4 - Problem 20.4 What alcohol is formed when each...Ch. 17.4 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 17.5 - Problem 20.8 Draw the products formed (including...Ch. 17.7 - Problem 20.10 Draw a stepwise mechanism for the...Ch. 17.7 - Prob. 10PCh. 17.7 - Problem 20.12 Draw the products formed from ...Ch. 17.7 - Prob. 12P
Ch. 17.7 - Prob. 13PCh. 17.8 - Prob. 14PCh. 17.8 - Problem-20.16 Review the oxidation reactions using...Ch. 17.9 - Problem-20.17 Write the step(s) needed to convert ...Ch. 17.9 - Prob. 18PCh. 17.10 - Problem 20.21 Draw the product of each reaction.
...Ch. 17.10 - Problem 20.22 Draw the products (including...Ch. 17.11 - Problem 20.23 What Grignard reagent and carbonyl...Ch. 17.11 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 17.11 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 17.12 - Prob. 24PCh. 17.13 - Problem 20.28 What ester and Grignard reagent are...Ch. 17.13 - Prob. 27PCh. 17.13 - Problem 20.30 What reagent is needed to convert ...Ch. 17.13 - Prob. 29PCh. 17.14 - What carboxylic acid formed from each alkyl halide...Ch. 17 - 20.37 Devise a synthesis of each alcohol from...Ch. 17 - 20.38 Draw the products formed when pentanal is...Ch. 17 - 20.39 Draw the product formed when is treated...Ch. 17 - The stereochemistry of the products of reduction...Ch. 17 - Prob. 40PCh. 17 - 20.42 Draw the products or each reduction...Ch. 17 - 20.44 Draw all stereoisomers formed in each...Ch. 17 - 20.54 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - 20.57 What ester and Grignard reagent are needed...Ch. 17 - 20.58 What organolithium reagent and carbonyl...Ch. 17 - 20.59 What epoxide and organometallic reagent are...Ch. 17 - Prob. 62PCh. 17 - 20.69 An unknown compound A (molecular formula )...Ch. 17 - 20.70 Treatment of compound C (molecular formula )...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Na :S f. F NO2arrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. + CI OH woཡི།༠w Br H مه D CI ပ။ Br H, Br Br H₂N OMe R IN Ill N S H CI Br CI CI D OH H 1/111arrow_forwardDraw the two products of the reaction. H₂C. CH₂ H :0: CH3 CH₂ +1arrow_forward
- 93 = Volume 93 = 5.32× 10 3 -23 ст a √ 1073 5.32× 10 3 cm³arrow_forwardASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 IZ IN Molecule 4 Molecule 5 ZI none of the above ☐ Molecule 3 Х IN www Molecule 6 NH Garrow_forwardHighlight each chiral center in the following molecule. If there are none, then check the box under the drawing area. There are no chiral centers. Cl Cl Highlightarrow_forwardA student proposes the following two-step synthesis of an ether from an alcohol A: 1. strong base A 2. R Is the student's proposed synthesis likely to work? If you said the proposed synthesis would work, enter the chemical formula or common abbreviation for an appropriate strong base to use in Step 1: If you said the synthesis would work, draw the structure of an alcohol A, and the structure of the additional reagent R needed in Step 2, in the drawing area below. If there's more than one reasonable choice for a good reaction yield, you can draw any of them. ☐ Click and drag to start drawing a structure. Yes No ロ→ロ 0|0 G Х D : ☐ பarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY