
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.2, Problem 2P
Which carbonyl groups in the anticancer drug taxol (Section
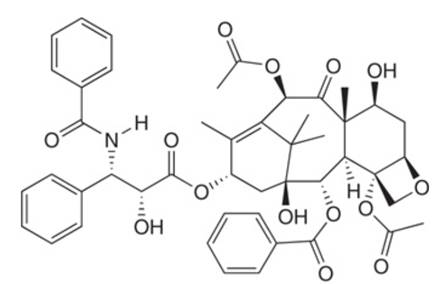
taxol
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 17 Solutions
Organic Chemistry (6th Edition)
Ch. 17.1 - Prob. 1PCh. 17.2 - Which carbonyl groups in the anticancer drug taxol...Ch. 17.2 - Prob. 3PCh. 17.4 - Problem 20.4 What alcohol is formed when each...Ch. 17.4 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 17.5 - Problem 20.8 Draw the products formed (including...Ch. 17.7 - Problem 20.10 Draw a stepwise mechanism for the...Ch. 17.7 - Prob. 10PCh. 17.7 - Problem 20.12 Draw the products formed from ...Ch. 17.7 - Prob. 12P
Ch. 17.7 - Prob. 13PCh. 17.8 - Prob. 14PCh. 17.8 - Problem-20.16 Review the oxidation reactions using...Ch. 17.9 - Problem-20.17 Write the step(s) needed to convert ...Ch. 17.9 - Prob. 18PCh. 17.10 - Problem 20.21 Draw the product of each reaction.
...Ch. 17.10 - Problem 20.22 Draw the products (including...Ch. 17.11 - Problem 20.23 What Grignard reagent and carbonyl...Ch. 17.11 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 17.11 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 17.12 - Prob. 24PCh. 17.13 - Problem 20.28 What ester and Grignard reagent are...Ch. 17.13 - Prob. 27PCh. 17.13 - Problem 20.30 What reagent is needed to convert ...Ch. 17.13 - Prob. 29PCh. 17.14 - What carboxylic acid formed from each alkyl halide...Ch. 17 - 20.37 Devise a synthesis of each alcohol from...Ch. 17 - 20.38 Draw the products formed when pentanal is...Ch. 17 - 20.39 Draw the product formed when is treated...Ch. 17 - The stereochemistry of the products of reduction...Ch. 17 - Prob. 40PCh. 17 - 20.42 Draw the products or each reduction...Ch. 17 - 20.44 Draw all stereoisomers formed in each...Ch. 17 - 20.54 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - 20.57 What ester and Grignard reagent are needed...Ch. 17 - 20.58 What organolithium reagent and carbonyl...Ch. 17 - 20.59 What epoxide and organometallic reagent are...Ch. 17 - Prob. 62PCh. 17 - 20.69 An unknown compound A (molecular formula )...Ch. 17 - 20.70 Treatment of compound C (molecular formula )...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License