
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 61P
What
a. 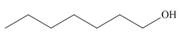 b.
b. 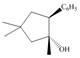 c.
c. 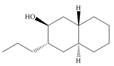
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Conduct a brief thermodynamic analysis of electrified interfaces. Gibbs model.
ELECTROCAPILAR EQUATION FOR IDEALLY POLARIZED ELECTRODES.
Briefly state the electrocapillary equation for ideally polarized electrodes.
Chapter 17 Solutions
Organic Chemistry (6th Edition)
Ch. 17.1 - Prob. 1PCh. 17.2 - Which carbonyl groups in the anticancer drug taxol...Ch. 17.2 - Prob. 3PCh. 17.4 - Problem 20.4 What alcohol is formed when each...Ch. 17.4 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 17.5 - Problem 20.8 Draw the products formed (including...Ch. 17.7 - Problem 20.10 Draw a stepwise mechanism for the...Ch. 17.7 - Prob. 10PCh. 17.7 - Problem 20.12 Draw the products formed from ...Ch. 17.7 - Prob. 12P
Ch. 17.7 - Prob. 13PCh. 17.8 - Prob. 14PCh. 17.8 - Problem-20.16 Review the oxidation reactions using...Ch. 17.9 - Problem-20.17 Write the step(s) needed to convert ...Ch. 17.9 - Prob. 18PCh. 17.10 - Problem 20.21 Draw the product of each reaction.
...Ch. 17.10 - Problem 20.22 Draw the products (including...Ch. 17.11 - Problem 20.23 What Grignard reagent and carbonyl...Ch. 17.11 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 17.11 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 17.12 - Prob. 24PCh. 17.13 - Problem 20.28 What ester and Grignard reagent are...Ch. 17.13 - Prob. 27PCh. 17.13 - Problem 20.30 What reagent is needed to convert ...Ch. 17.13 - Prob. 29PCh. 17.14 - What carboxylic acid formed from each alkyl halide...Ch. 17 - 20.37 Devise a synthesis of each alcohol from...Ch. 17 - 20.38 Draw the products formed when pentanal is...Ch. 17 - 20.39 Draw the product formed when is treated...Ch. 17 - The stereochemistry of the products of reduction...Ch. 17 - Prob. 40PCh. 17 - 20.42 Draw the products or each reduction...Ch. 17 - 20.44 Draw all stereoisomers formed in each...Ch. 17 - 20.54 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - 20.57 What ester and Grignard reagent are needed...Ch. 17 - 20.58 What organolithium reagent and carbonyl...Ch. 17 - 20.59 What epoxide and organometallic reagent are...Ch. 17 - Prob. 62PCh. 17 - 20.69 An unknown compound A (molecular formula )...Ch. 17 - 20.70 Treatment of compound C (molecular formula )...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forward
- a. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forwardGiven the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forward
- Electrochemistry. State the difference between E and E0.arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY