
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 59P
What ester and Grignard reagent are needed to synthesize each alcohol?
a. 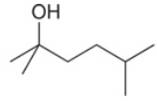 b.
b. 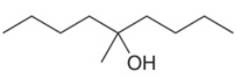
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Finish the reactions
hand written please
Part A
Identify each alcohol as primary, secondary, or tertiary
Drag the appropriate items to their respective bins.
CH₂
H₂C-
-C-OH
HO
CH₂
Primary
Он
OH
CH₂
OH
CCH₂OH
CH₂
сн
Secondary
Tertiary
Reset Help
CH,CH₂
(CH)CHCH,OH CH,CH,CH,CCH,
CHOH
CH₂
Different types of alcohol groups
Alcohol and its reaction:
8. Combing two alcohol molecules below and completing the reaction with
Product .( Hint Reaction called etherification as ether is formed and name the
ether once you complete the reaction.
Hint.:
R-O-H+H-O-RR-O-R
Do the reaction:
CH₂OH + CH₂OH---→
+ H-O-H
9. Write the reaction of formation of alcohol from alkene by adding water:
Addition reaction also called hydration reaction as we are adding water
which occur always in presence of acid
Hint: Break the double bond and add H and OH if symmetrical then
add anywhere if unsymmetrical then follow Markovnikov rule H
should go to that double bone carbon which has more hydrogen
CH2=CH2 + H₂O-→
Complete the reaction
hand written please
Chapter 17 Solutions
Organic Chemistry (6th Edition)
Ch. 17.1 - Prob. 1PCh. 17.2 - Which carbonyl groups in the anticancer drug taxol...Ch. 17.2 - Prob. 3PCh. 17.4 - Problem 20.4 What alcohol is formed when each...Ch. 17.4 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 17.5 - Problem 20.8 Draw the products formed (including...Ch. 17.7 - Problem 20.10 Draw a stepwise mechanism for the...Ch. 17.7 - Prob. 10PCh. 17.7 - Problem 20.12 Draw the products formed from ...Ch. 17.7 - Prob. 12P
Ch. 17.7 - Prob. 13PCh. 17.8 - Prob. 14PCh. 17.8 - Problem-20.16 Review the oxidation reactions using...Ch. 17.9 - Problem-20.17 Write the step(s) needed to convert ...Ch. 17.9 - Prob. 18PCh. 17.10 - Problem 20.21 Draw the product of each reaction.
...Ch. 17.10 - Problem 20.22 Draw the products (including...Ch. 17.11 - Problem 20.23 What Grignard reagent and carbonyl...Ch. 17.11 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 17.11 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 17.12 - Prob. 24PCh. 17.13 - Problem 20.28 What ester and Grignard reagent are...Ch. 17.13 - Prob. 27PCh. 17.13 - Problem 20.30 What reagent is needed to convert ...Ch. 17.13 - Prob. 29PCh. 17.14 - What carboxylic acid formed from each alkyl halide...Ch. 17 - 20.37 Devise a synthesis of each alcohol from...Ch. 17 - 20.38 Draw the products formed when pentanal is...Ch. 17 - 20.39 Draw the product formed when is treated...Ch. 17 - The stereochemistry of the products of reduction...Ch. 17 - Prob. 40PCh. 17 - 20.42 Draw the products or each reduction...Ch. 17 - 20.44 Draw all stereoisomers formed in each...Ch. 17 - 20.54 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - 20.57 What ester and Grignard reagent are needed...Ch. 17 - 20.58 What organolithium reagent and carbonyl...Ch. 17 - 20.59 What epoxide and organometallic reagent are...Ch. 17 - Prob. 62PCh. 17 - 20.69 An unknown compound A (molecular formula )...Ch. 17 - 20.70 Treatment of compound C (molecular formula )...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forward
- Indicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY