
(a)
Interpretation:
Highlight the aldehyde or ketone group in given compounds.
Concept Introduction:
Both
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
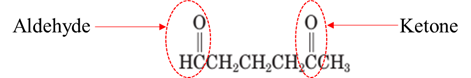
Explanation of Solution
Both aldehydes and ketones contain
(b)
Interpretation:
Highlight the aldehyde or ketone group in the given compound.
Concept Introduction:
Both aldehydes and ketones contain
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
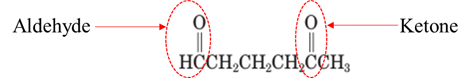
Explanation of Solution
Both aldehydes and ketones contain a
(c)
Interpretation:
Highlight the aldehyde or ketone group in the given compound.
Concept Introduction:
Both aldehydes and ketones contain
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
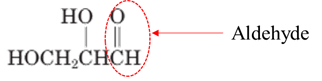
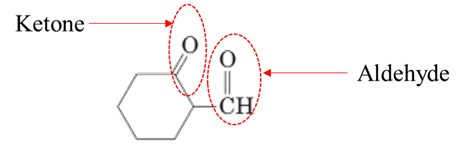
Explanation of Solution
Both aldehydes and ketones contain a

(d)
Interpretation:
Highlight the aldehyde or ketone group in the given compound.
Concept Introduction:
Both aldehydes and ketones contain
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
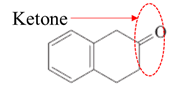
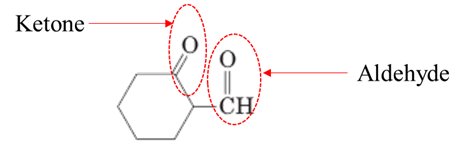
Explanation of Solution
Both aldehydes and ketones contain a
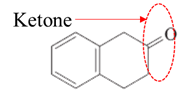
(e)
Interpretation:
Highlight the aldehyde or ketone group in the given compound.
Concept Introduction:
Both aldehydes and ketones contain
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
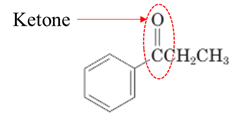
Explanation of Solution
Both aldehydes and ketones contain a
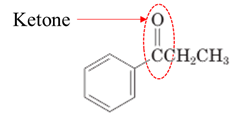
(f)
Interpretation:
Highlight the aldehyde or ketone group in the given compound.
Concept Introduction:
Both aldehydes and ketones contain
Here, r is alkyl group.
The general formula of ketone group is as follows:
Here, r and
Answer to Problem 17.64P
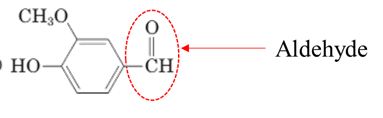
Explanation of Solution
Both aldehydes and ketones contain a
Want to see more full solutions like this?
Chapter 17 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Please help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship. I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?arrow_forwardDescribe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.arrow_forwardDon't used hand raitingarrow_forward
- It is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forwardPlease draw the inverted chair forms of the products for the two equilibrium reactions shown below. Circle the equilibrium reaction that would have a AG = 0, i.e., the relative energy of the reactant (to the left of the equilibrium arrows) equals the relative energy of the product? [No requirement to show or do calculations.] CH3 CH3 HH CH3 1 -CH3arrow_forward
- 5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forwardThe mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward
- 32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forwardDon't used hand raitingarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



