
(a)
Interpretation:
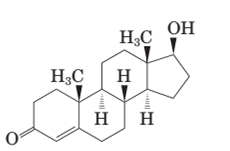
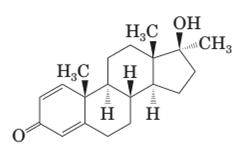
The differences in the structural formula of testosterone and methandrostenolone should be determined along with
Concept Introduction:
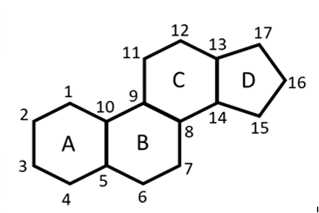
Steroids are the third major class of lipids. These are the compounds containing the following ring system:
Answer to Problem 17.76P
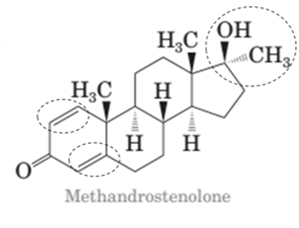
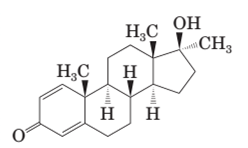
Both methandrostenolone and testosterone hormone contains hydroxyl functional group (alcohol) and carbonyl functional group (kentone).
In the structural formula of testosterone hormone, there is only one double bond and it has only one methyl group attached to cyclopentane ring whereas in methandrostenolone there are two double bonds and two methyl groups are attached to the cyclopentane ring.
Explanation of Solution
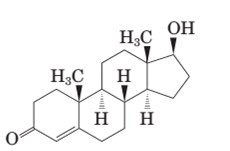
Testosterone and methandrostenolone both the hormones contain one −OH group attached to cyclopentane ring and one >C=O group in cyclohexene ring. Therefore both alcoholic and
In the structure of testosterone there is only a double bond in cyclohexene ring having ketonic group and no methyl group is present on the carbon of cyclopentane ring having alcoholic group as shown:
In the structure of methandrostenolone, there are two double bonds in cyclohexene ring having ketonic group and one methyl group attached on the carbon of cyclopentane ring having alcoholic group as shown:
(b)
Interpretation:
In both the given hormones mark all the stereocenters and state the number of stereoisomer possible for each.
Concept Introduction:
Stereoisomers are the compounds that are differ only in the spatial arrangement of their atoms. Each stereoisomer has at least one stereocenter. A stereocenter is a tetrahedral carbon atom that has four different groups bonded to it. For a molecule with n sterocenters, a maximum of
Answer to Problem 17.76P
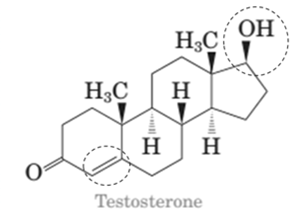
Stereocenters in testosterone:
Since testosterone has six stereocenters therefore it has 64 possible stereoisomers.
Stereocenters in methandrostenolone:
Since methandrostenolone has six stereocenters therefore it has 64 possible stereoisomers.
Explanation of Solution
A stereocenter is a tetrahedral carbon atom that has four different groups bonded to it.
Stereocenters in testosterone:
In testosterone there are six stereocenters.
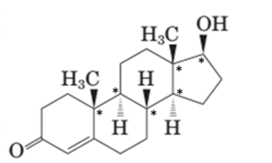
For a molecule with n stereocenter, a maximum of
Stereocenters in methandrostenolone:
In methandrostenolone there are six stereocenters.
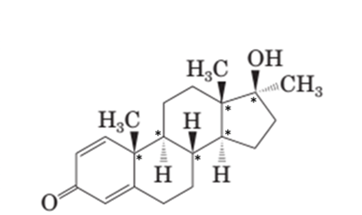
For a molecule with n stereocenter, a maximum of
Want to see more full solutions like this?
Chapter 17 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- 20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward20.21 Predict the major product formed by 1,4-addition of HCI to cyclopentadiene. 20.22 Draw structural formulas for the two constitutional isomers with the molecular for- mula C₂H,Br, formed by adding one mole of Br, to cyclopentadiene.arrow_forward
- Add substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward+ Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. Br Drawing Strong Base H Q Atoms, Bonds Charges and Rings Draw or tap a new bond to see suggestions. Remove Done 語 Reset Undo + Drag To Panarrow_forward
- Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. + Drawing Į Strong Base H Br Q Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset 謂 Remove Done Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. + Br CH3 Q Strong Base Drawing Atoms, Bonds and Rings Charges Undo Reset H "Br H N Br. Remove Done .N. Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this elementary step in an elimination mechanism. Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore byproducts. + Br: .. 8 0.01 M NaOH heat Drawing Q Atoms, Bonds and Rings Charges and Lone Pairs Draw or tap a new bond to see suggestions. Undo Reset Remove Done + Drag To Panarrow_forward
- + Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. Ph CH2CH3 H H3C H Br DBN [૪] Drawing Atoms, Bonds and Rings H | OH Charges ―00 H. C | Undo Reset Br I Remove Done Drag To Pan +arrow_forwardReaction A Now the production A Œ In the product of reaction i 12 Dear the product of actionarrow_forwardMacmillan Learnin When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm-1. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 DEPT 135 200 160 120 80 40 0 Draw the unknown amide. 40 40 0arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

