
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.5, Problem 16.45PP
The following graph shows the activity versus pH curves for pepsin, sucrose, and trypsin. Estimate the optimum pH for each.
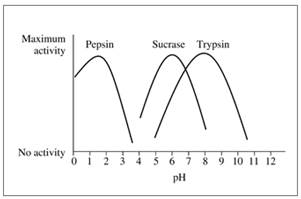
16.46 Refer to the graph in problem 16.45 to determine if the reaction rate in each condition will be at the optimum rate or not.
- Trypsin, pH 5.0
- Sucrase, pH 5.0
- Pepsin, pH 4.0
- Trypsin, pH 8.0
- Pepsin, pH 2.0
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Solve this
く
Predicting the pr
Predict the major products of the following organic reaction:
Δ
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
?
Click and drag to start drawing a structure.
propose synthesis
Chapter 16 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 16.1 - Classify each of the following proteins according...Ch. 16.1 - Prob. 16.2PPCh. 16.1 - Prob. 16.3PPCh. 16.1 - Prob. 16.4PPCh. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the strcture for each of the following amino...Ch. 16.1 - Prob. 16.8PPCh. 16.1 - Prob. 16.9PPCh. 16.1 - Prob. 16.10PP
Ch. 16.2 - Prob. 16.11PPCh. 16.2 - Prob. 16.12PPCh. 16.2 - Prob. 16.13PPCh. 16.2 - Prob. 16.14PPCh. 16.2 - Prob. 16.15PPCh. 16.2 - Prob. 16.16PPCh. 16.3 - Prob. 16.17PPCh. 16.3 - Prob. 16.18PPCh. 16.3 - Prob. 16.19PPCh. 16.3 - Prob. 16.20PPCh. 16.3 - What type of interaction would you expect between...Ch. 16.3 - What type of interaction would you expect between...Ch. 16.3 - Prob. 16.23PPCh. 16.3 - Prob. 16.24PPCh. 16.3 - Prob. 16.25PPCh. 16.3 - Prob. 16.26PPCh. 16.3 - Prob. 16.27PPCh. 16.3 - Indicate the changes in secondary and tertiary...Ch. 16.4 - Why do chemical reactions in the body require...Ch. 16.4 - Prob. 16.30PPCh. 16.4 - Prob. 16.31PPCh. 16.4 - Prob. 16.32PPCh. 16.4 - Prob. 16.33PPCh. 16.4 - Prob. 16.34PPCh. 16.4 - Prob. 16.35PPCh. 16.4 - 16.36 Match the terms (1) active site, (2)...Ch. 16.4 - Prob. 16.37PPCh. 16.4 - Prob. 16.38PPCh. 16.4 - Prob. 16.39PPCh. 16.4 - Prob. 16.40PPCh. 16.4 - For problems 16.39 to 16.42, see Chemistry Link to...Ch. 16.4 - Prob. 16.42PPCh. 16.5 - Trypsin, a peptidase that hydrolyzes polypeptides,...Ch. 16.5 - pepsin, a peptidase that hydrolyzes proteins,...Ch. 16.5 - The following graph shows the activity versus pH...Ch. 16.5 - The following graph shows the activity versus pH...Ch. 16.5 - Prob. 16.47PPCh. 16.5 - Prob. 16.48PPCh. 16.5 - Prob. 16.49PPCh. 16.5 - Prob. 16.50PPCh. 16.5 - What is the chemical formula for hydroxyurea?Ch. 16.5 - What is the molar mass of hydroxyurea?Ch. 16.5 - Prob. 16.53PPCh. 16.5 - Prob. 16.54PPCh. 16 - Prob. 16.55UTCCh. 16 - Prob. 16.56UTCCh. 16 - Prob. 16.57UTCCh. 16 - Prob. 16.58UTCCh. 16 - 16.59 Identify the amino acids and type of...Ch. 16 - What type of interaction would you expect between...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Seed and vegetables are often deficient in one or...Ch. 16 - 16.64 Seeds and vegetables are often deficient in...Ch. 16 - Prob. 16.65APPCh. 16 - Prob. 16.66APPCh. 16 - Prob. 16.67APPCh. 16 - Prob. 16.68APPCh. 16 - Prob. 16.69APPCh. 16 - Why do enzymes function only under mild...Ch. 16 - Prob. 16.71APPCh. 16 - Prob. 16.72APPCh. 16 - Prob. 16.73APPCh. 16 - Prob. 16.74APPCh. 16 - Prob. 16.75APPCh. 16 - Prob. 16.76APPCh. 16 - Prob. 16.77APPCh. 16 - Prob. 16.78APPCh. 16 - If a blood test indicates a high level of LDH and...Ch. 16 - Prob. 16.80APPCh. 16 - Prob. 16.81CPCh. 16 - Prob. 16.82CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explanation O Conjugated Pi Systems Deducing the reactants of a Diels-Alder reaction Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Xarrow_forwardDiels Alder Cycloaddition: Focus on regiochemistry (problems E-F) –> match + of thedienophile and - of the diene while also considering stereochemistry (endo).arrow_forwardHELP! URGENT! PLEASE RESOND ASAP!arrow_forward
- Question 4 Determine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267 First-order, k = 0.210 hour 1 First-order, k = 0.0912 hour 1 O Second-order, k = 0.590 M1 hour 1 O Zero-order, k = 0.0770 M/hour O Zero-order, k = 0.4896 M/hour O Second-order, k = 1.93 M-1-hour 1 10 ptsarrow_forwardDetermine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267arrow_forwardDraw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry. Ignore inorganic byproducts. OSO4 (cat) (CH3)3COOH Select to Draw ઘarrow_forward
- Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward
- 2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forwardSteps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY