
Concept explainers
a)
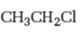
Interpretation:
Whether ethyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an
To state and explain:
Whether ethyl chloride is expected to undergo Friedal- Crafts reaction with or without rearrangement.
b)
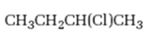
Interpretation:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift (particularly when a primary alkyl halide is used) can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
c)
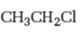
Interpretation:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement.
d)
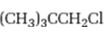
Interpretation:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
e)
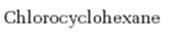
Interpretation:
Whether chlorocyclohexane is expected to undergo Friedal Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to yield an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether chlorocyclohexane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
Trending nowThis is a popular solution!

Chapter 16 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Label the α and ẞ carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K-OC(CH3), b. ان Brarrow_forwardSuppose a reaction has the following mechanism:A + B → C + D C + C → F F + B → A + A + GIt is known that C is a reaction intermediate. Of the following options, indicate which are true:1. The overall reaction could be 3B → 2D + G.2. A could be a catalyst.3. C is the only intermediate that can exist.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





