
a)
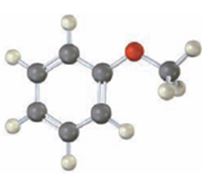
Interpretation:
Draw the products obtained when the compound shown (methoxybenzene) reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Concept introduction:
Aromatic rings can be brominated using Br2 in the presence of FeBr3. The electrophile is Br+. Friedal-Crafts acylation reaction occurs when an
To draw:
The products obtained when the compound shown (methoxybenzene) reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Answer to Problem 24VC
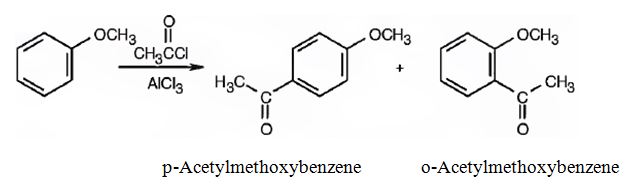
(i) The products obtained when the compound shown (methoxybenzene) reacts with Br2/FeBr3 are p-bromomethoxybenzene and o-bromomethoxybenzene.
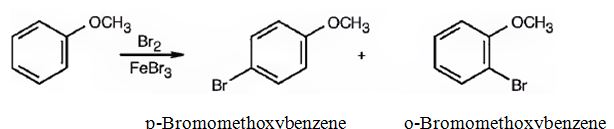
(ii) The products obtained when the compound shown (methoxybenzene) reacts with CH3COCl/AlCl3 are p-acetylmethoxybenzene and o-acetylmethoxybenzene.
Explanation of Solution
The methoxy group attached to the benzene ring is an ortho & para directing group. When methoxybenzene is brominated and alkylated it directs the incoming electrophiles, Br+ and CH3CO+ to ortho & para positions.
(i) The products obtained when the compound shown (methoxybenzene) reacts with Br2/FeBr3 are p-bromomethoxybenzene and o-bromomethoxybenzene.

(ii) The products obtained when the compound shown (methoxybenzene) reacts with CH3COCl/AlCl3 are p-acetylmethoxybenzene and o-acetylmethoxybenzene.

b)
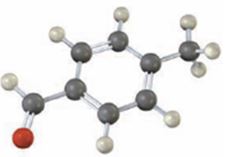
Interpretation:
Draw the structures of the products obtained when the compound shown, p-methylbenzaldehyde reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Concept introduction:
Electrophilic substitution of disubstituted benzenes follows three simple rules. (i) If the directing influence of both the substituents reinforce each other, a single product results. (ii) If the directing influences of both the substituent groups oppose each other, the most powerful activating group among them has the dominant influence but usually a mixture of products results. (iii) In meta disubstituted compounds, further substitution in between the groups occurs only rarely, due to steric reasons.
To show:
The major product(s) produced when p-methylbenzaldehyde reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Answer to Problem 24VC
(i) The major product produced when p-methylbenzaldehyde reacts with Br2/FeBr3 is 3-bromo-4-methylbenzaldehyde.
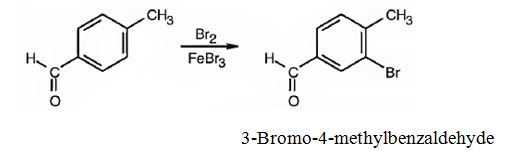
(ii) The major product produced when p-methylbenzaldehyde reacts with CH3COCl/AlCl3 is 3-acetyl-4-methylbenzaldehyde.
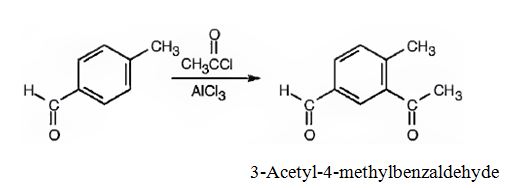
Explanation of Solution
The
(i) The major product produced when p-methylbenzaldehyde reacts with Br2/FeBr3 is 3-bromo-4-methylbenzaldehyde.

(ii)The major product produced when p-methylbenzaldehyde reacts with CH3COCl/AlCl3 is 3-acetyl-4-methylbenzaldehyde.

Want to see more full solutions like this?
Chapter 16 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


