
Use the diagram below to answer Questions 8 and 9.
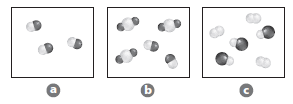
Which sample could contain particles of magnesiumfluoride?
A. a
B. b
C. c
D. Both a and b
Interpretation:
The sample that contain particles a of magnesium fluoride from the given figure needs to be determined.
 Concept introduction:
Concept introduction:
Diatomic molecules consists two atoms of different or same elements. For example,
Answer to Problem 9STP
B.b
Explanation of Solution
B. magnesium fluoride has three atoms - one magnesium and two fluorine atoms. Two fluorine atoms are attached to the magnesium atom. The given figure shows different size and colors. Option B has three atoms which attached to each other with larger central atom and two atoms are the same in color.
The incorrect options are as follows:
A. a
Figure a has different atoms that attached one another.
C. c
Figure c has different atoms and two identical atoms that attached one another.
D. both a and b
Both figure a has different atoms.
Chapter 16 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: A Molecular Approach (4th Edition)
Biology: Life on Earth (11th Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
- Which of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forwardIndicate the order of basicity of primary, secondary and tertiary amines.arrow_forward> Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. Cl Z- N O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic ○ antiaromatic nonaromaticarrow_forward
- Please help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





