
Interpretation:
To identify the labels in the given figure.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
Answer to Problem 72A
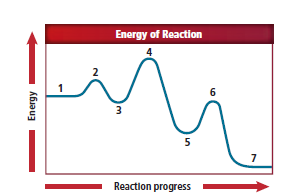
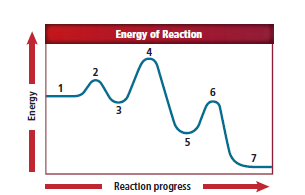
In figure 16.23, label 1 represents the reactants, label 2 represents the activated complex, label 3 represents intermediate step, label 4 represents activated complex., label 5 represents products and label 6 also represents the activated complex.
Explanation of Solution
From the figure,
Each step signifies a series of elementary reactions. The reaction begins with the reactants and proceeds through an intermediate to generate products.
Label 1 represents the reactants as it is located at the beginning of the reaction.
Label 2 represents an activated complex because it is located just after label 1.
Label 3 represents the intermediate step because the curve in this location is lower in energy than that of the activated complex.
The intermediate which is produced from the first elementary step used as reactants in the second elementary step. An activated complex is generated when the intermediates react.
This particular elementary step includes an activated complex with a higher energy than in the previous elementary step. Label 4 represents the activated complex.
The products are formed after the activated complex briefly occurs in the second elementary step. Label 5 represents the products. The products located in label 5 is lower in energy than that of the activated complex.
The intermediate that is produced from the second elementary step is used as a reactant in the third elementary step. These intermediates react to generate an activated complex. Label 6 represents the activated complex.
Label 1 represents reactants, label 2 represents activated complex, label 3 represents the intermediate step, label 4 represents the activated complex, label 5 represents the products and label 6 represents the activated complex
Chapter 16 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
Campbell Biology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Campbell Essential Biology (7th Edition)
- Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forwardWhat is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forward
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





