1 Chemistry: The Central Science 2 Atoms, Molecules, And Ions 3 Stoichiometry: Ratios Of Combination 4 Reactions In Aqueous Solutions 5 Thermochemistry 6 Quantum Theory And The Electronic Structure Of Atoms 7 Electron Configuration And The Periodic Table 8 Chemical Bonding I: Basic Concepts 9 Chemical Bonding Ii: Molecular Geometry And Bonding Theories 10 Gases 11 Intermolecular Forces And The Physical Properties Of Liquids And Solids 12 Modern Materials 13 Physical Properties Of Solutions 14 Chemical Kinetics 15 Chemical Equilibrium 16 Acids And Bases 17 Acid-base Equilibria And Solubility Equilibria 18 Entropy, Free Energy, And Equilibrium 19 Electrochemistry 20 Nuclear Chemistry 21 Environmental Chemistry 22 Coordination Chemistry 23 Metallurgy And The Chemistry Of Metals 24 Nonmetallic Elements And Their Compounds 25 Organic Chemistry expand_more
16.1 Sample Problem And Checkpoint 16.2 Sample Problem And Checkpoint 16.3 Sample Problem And Checkpoint 16.4 Sample Problem And Checkpoint 16.5 Sample Problem And Checkpoint 16.6 Sample Problem And Checkpoint 16.7 Sample Problem And Checkpoint 16.8 Sample Problem And Checkpoint 16.9 Sample Problem 16.10 Sample Problem And Checkpoint 16.11 Sample Problem 16.12 Sample Problem And Checkpoint 16.13 Sample Problem 16.14 Sample Problem 16.15 Sample Problem 16.16 Sample Problem 16.17 Sample Problem 16.18 Sample Problem 16.19 Sample Problem 16.20 Sample Problem 16.21 Sample Problem 16.22 Sample Problem 16.23 Sample Problem Chapter Questions expand_more
Problem 1KSP: Calculate the pH of a solution that is 0.22 M in nitrite ion (NO 2 − ) at 25°C . K a for nitrous... Problem 2KSP: 16.2 Determine pH at the equivalence point in the titration of 41.0 mL 0.096 M formic acid with... Problem 3KSP: Calculate the pH of a solution that is 0.22 M in pyridinium ion ( C 5 H 5 NH - ) at 25°C . K b for... Problem 4KSP: 16.4 Determine pH at the equivalence point in the titration of 26.0 mL 1.12 M pyridine with
(a)... Problem 1QP: Define Brønsted acids and bases. Give an example of a conjugate pair in an acid-base reaction. Problem 2QP: For a species to act as a Brønsted base, an atom in the species must possess a lone pair of... Problem 3QP: 16.3 Classify each of the following species as a Brønsted acid or base, or both: (a) (b) (c) (d) ... Problem 4QP: Identify the acid-base conjugate pairs in each of the following reactions: (a) C H 3 C O O − + H C N... Problem 5QP: 16.5 Write the formulas of the conjugate bases of the following acids: (formic acid).
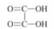
Problem 6QP: Write the formula for the conjugate acid of each of the following bases: ( a ) H S − , ( b ) H C O 3... Problem 7QP: Which of the following could represent a Brønsted acid-base reaction? Problem 8QP: 16.8 Oxalic acid has the following structure:
An oxalic acid solution contains the following... Problem 9QP: Rite the equilibrium expression for the autoionization of water and an equation relating [ H 3 O - ]... Problem 10QP: 16.10 In Section 15.3 we learned that when we multiply a chemical equation by 2, we must square its... Problem 11QP: 16.11 The equilibrium constant for the autoionization of water:
is . Is the forward process... Problem 12QP: 16.12 Define the term amphoteric.
Problem 13QP: 16.13 Compare the magnitudes of in aqueous solutions that are acidic, basic, and neutral.
Problem 14QP: Calculate the OH - concentration in an aqueous solution at 25°C with each of the following H 3 O -... Problem 15QP: 16.15 Calculate the concentration in an aqueous solution at with each of the following ... Problem 16QP: The value of K w at 50°C is 5.48 × 10 − 14 . Calculate the OH- concentration in each of the aqueous... Problem 17QP: The value of K w at 100°C is 5.1 × 3 10 − 13 . Calculate the H 3 O - concentration in each of the... Problem 18QP Problem 19QP Problem 20QP Problem 21QP Problem 22QP Problem 23QP: Calculate the concentration of OH- ions in a 1 .4 ×10 -3 M HCl solution. Problem 24QP Problem 25QP: 16.25 Calculate the pH of each of the following solutions: (a) 0.0010 M , (b) 0.76 M KOH.
Problem 26QP: Calculate the pH of each of the following solutions: ( a ) 2 .8 × 10 -4 M Ba ( OH ) 2 , ( b ) 5 .2 ×... Problem 27QP Problem 28QP Problem 29QP: 16.29 The pOH of a solution is 9.40 at . Calculate the hydronium ion concentration of the solution.
Problem 30QP Problem 31QP Problem 32QP: 16.32 A solution is made by dissolving 18.4 g of in enough water to make 662 mL of solution.... Problem 33QP Problem 34QP Problem 35QP Problem 36QP Problem 37QP Problem 38QP Problem 39QP Problem 40QP: 16.40 Calculate the concentration of in a solution at that has a .
Problem 41QP: Calculate the concentration of HNO 3 in a solution at 25°C that has a pH of (a) 4.21, (b) 3.55, and... Problem 42QP Problem 43QP Problem 44QP Problem 45QP Problem 46QP: Explain what is meant by the strength of an acid. Problem 47QP Problem 48QP Problem 49QP: Why do we normally not quote K a values for strong acids such as HCl and HNO 3 ? Why is it necessary... Problem 50QP: Which of the following solutions has the highest pH: (a) 0 .40 M HCOOH, (b) 0 .40 M HClO 4 , (c) 0... Problem 51QP: Without referring to the text, write the formulas of four weak acids. Problem 52QP: In biological and medical applications, it is often necessary to study the autoionization of water... Problem 53QP: 16.53 The for benzoic acid is Calculate the pH of a 0.10-M aqueous solution of benzoic acid at .
Problem 54QP: The K a for hydrofluoric acid is 7.1 × 10 − 4 . Calculate the pH of a 0.15-M aqueous solution of... Problem 55QP: Calculate the pH of an aqueous solution at 25°C that is 0.095 M in hydrocyanic acid (HCN). ( K a for... Problem 56QP: Calculate the pH of an aqueous solution at 25°C that is 0.34 M in phenol ( C 6 H 5 OH ) . (K a for... Problem 57QP: 16.57 Determine the percent ionization of the following solutions of formic acid at
Problem 58QP Problem 59QP Problem 60QP Problem 61QP: Calculate the K a of a weak acid if a 0.19 − M aqueous solution of the acid has a pH of 4 .52 at... Problem 62QP Problem 63QP: What is the original molarity of a solution of formic acid ( HCOOH ) whose pH is 3.26 at ? (25°C? K... Problem 64QP: What is the original molarity of a solution of a weak acid whose K a is 3 .5 ×10 -5 and whose pH is... Problem 65QP: 16.65 Which of the following statements are true for a solution of a weak acid HA? (Choose all that... Problem 66QP Problem 67QP Problem 68QP: Compare the pH values for 0.10 − M solutions of NaOH and of NH 3 to illustrate the difference... Problem 69QP: Which of the following has a higher pH: (a) 1 .0 M NH 3 , (b) 0 .20 M NaOH ( K b for NH 3 =1 .8×10... Problem 70QP Problem 71QP: The pH of a 0.30-M solution of a weak base is 10.66 at 25°C . What is the K b of the base? Problem 72QP: What is the original molarity of an aqueous solution of ammonia (NH 3 ) whose pH is 11.22 at 25°C (... Problem 73QP Problem 74QP Problem 75QP Problem 76QP Problem 77QP Problem 78QP Problem 79QP: 16.79 Calculate for each of the following ions: (See Table 16.6.)
Problem 80QP Problem 81QP Problem 82QP Problem 83QP Problem 84QP Problem 85QP: Compare the pH of a 0 .040 M HCl solution with that of a 0.040 M H 2 SO 4 solution. (Hint: H 2 SO 4... Problem 86QP: What are the concentrations of HSO 4, – SO 2– 4 , and H 3 O – in a 0 .20 M KHSO 4 solution? (Hint: H... Problem 87QP: 16.87 Calculate the concentrations of
Problem 88QP: Calculate the pH at 25°C of a 0.25 − M aqueous solution of phosphoric acid ( H 3 PO 4 ) . ( K a 1 ,... Problem 89QP: 16.89 Calculate the pH at of a aqueous solution of oxalic acid . (, and , for oxalic acid are ,... Problem 90QP: The first and second ionization constants of a diprotic acid H 2 A are K a 1 and K a 2 at a certain... Problem 91QP Problem 92QP Problem 93QP Problem 94QP Problem 95QP Problem 96QP Problem 97QP Problem 98QP: Define salt hydrolysis. Categorize salts according to how they affect the pH of a solution. Problem 99QP: 16.99 Explain why small, highly charged metal ions are able to undergo hydrolysis.
Problem 100QP: Al 3+ is not a Brønsted acid, but Al( H 2 O ) 6 3+ is. Explain. Problem 101QP: Specify which of the following salts will undergo hydrolysis: KF, NaN O 3 , N H 4 N O 2 , MgS O 4 ,... Problem 102QP Problem 103QP: Calculate the pH of a 0 .42 M NH 4 Cl solution . ( K b for ammonia = 1 .8 × 1 0 -5 .) Problem 104QP Problem 105QP Problem 106QP Problem 107QP: 16.107 Predict whether the following solutions are acidic, basic, or nearly neutral:
Problem 108QP: A certain salt, MX (containing the M + and X - ions), is dissolved in water, and the pH of the... Problem 109QP Problem 110QP: Predict whether a solution containing the salt K 2 HPO 4 will be acidic, neutral, or basic. Problem 111QP Problem 112QP Problem 113QP Problem 114QP Problem 115QP Problem 116QP Problem 117QP Problem 118QP Problem 119QP Problem 120QP Problem 121QP Problem 122QP Problem 123QP Problem 124QP Problem 125QP: Identity the Lewis acid and the Lewis base in the following reactions: (a) AlBr 3 ( s ) + Br - ( a q... Problem 126AP: Predict the direction that predominates in this reaction: F - ( a q ) + H 2 O ( l ) ⇄ HF ( a q ) +... Problem 127AP Problem 128AP Problem 129AP: Calculate the pH and percent ionization of a 0 .88 M HNO 2 solution at 25°C . Problem 130AP: 16.130 Calculate the pH of a 0.20 M ammonium acetate solution.
Problem 131AP Problem 132AP Problem 133AP: 16.133 Like water, liquid ammonia undergoes autoionization:
(a) Identify the Brønsted acids and... Problem 134AP Problem 135AP: A solution contains a weak monoprotic acid HA and its sodium salt NaA both at o.1 M concentration.... Problem 136AP Problem 137AP Problem 138AP Problem 139AP Problem 140AP: A 10.0-g sample of white phosphorus was burned in an excess of oxygen. The product was dissolved in... Problem 141AP Problem 142AP Problem 143AP Problem 144AP Problem 145AP: 16.145 Give an example of (a) a weak acid that contains oxygen atoms, (b) a weak acid that does not... Problem 146AP Problem 147AP Problem 148AP Problem 149AP: When chlorine reacts with water, the resulting solution is weakly acidic and reacts with NaOH to... Problem 150AP Problem 151AP: Calculate the pH of a 2 .00 M NH 4 CN solution. Problem 152AP: Calculate the concentrations of all species in a 0 .100 M H 3 PO 4 solution. Problem 153AP Problem 154AP: 16.154 Calculate the concentrations of all the species in a solution.
Problem 155AP Problem 156AP: Calculate the pH of a solution that is 1.00 M HCN and 1.00 M HF. Compare the concentration (in... Problem 157AP: How many grams of NaCN would you need to dissolve in enough water to make exactly 250 mL of solution... Problem 158AP: A solution of formic acid ( HCOOH ) has a pH of 2.53. How many grams of formic acid are there in... Problem 159AP: Calculate the pH of a 1-L solution containing 0.150 mole of CH 3 COOH and 0.100 mole of HCl . Problem 160AP: 16.160 A 1.87-g sample of Mg reacts with 80.0 mL of a solution whore pH is -0.544. What is the pH... Problem 161AP Problem 162AP Problem 163AP: A 0.400 M formic acid ( HCOOH ) solution freezes at -0 .758ºC . Calculate the K a of the acid at... Problem 164AP Problem 165AP Problem 166AP Problem 167AP: 16.167 Both the amide ion and the nitride ion are stronger bases than the hydroxide ion and hence... Problem 168AP: Determine whether each of the following statements is true or false. If false, explain why the... Problem 169AP Problem 170AP Problem 171AP Problem 172AP: 16.172 A typical reaction between an antacid and the hydrochloric acid in gastric juice... Problem 173AP Problem 174AP: 16.174 Hemoglobin is a blood protein that is responsible for transporting oxygen. It can exist in... Problem 175AP: Tooth enamel is largely hydroxyapatite [ Ca 3 ( PO 4 ) 3 OH ] . When it dissolves in water (a... Problem 176AP Problem 177AP Problem 178AP: About half of the hydrochloric acid produced annually in the United States (3.0 billion pounds) is... Problem 179AP Problem 180AP Problem 181AP Problem 182AP: (a) Use VSEPR to predict the geometry of the hydronium ion ( H 3 O + ) . (b) The O atom in H 2 O has... Problem 1SEPP: The following questions are not based on a descriptive passage. Which of the following best... Problem 2SEPP: The following questions are not based on a descriptive passage. What is the pH of a solution made by... Problem 3SEPP: The following questions are not based on a descriptive passage.
3. What volume of 0.085 M is... Problem 4SEPP: The following questions are not based on a descriptive passage.
4. Determine the pH at the... format_list_bulleted


 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
