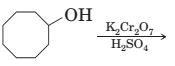Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357391594
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 72P
17-78 Complete the following equation for these reactions.
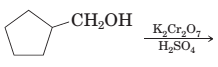
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Answe Answer A and B please
3. Refer to the data below to answer the following questions:
Isoelectric point
Amino Acid
Arginine
10.76
Glutamic Acid
3.22
Tryptophan
5.89
A. Define isoelectric point.
B. The most basic amino acid is
C. The most acidic amino acid is
sidizo zo
3. A gas mixture contains 50 mol% H2 and 50 mol% He.
1.00-L samples of this gas mixture are mixed with
variable volumes of O2 (at 0 °C and 1 atm). A spark is
introduced to allow the mixture to undergo complete
combustion. The final volume is measured at 0 °C and 1
atm. Which graph best depicts the final volume as a
function of the volume of added O2?
(A)
2.00
1.75
Final Volume, L
1.50
1.25
1.00
0.75
0.50
0.25
0.00
0.00
0.25
0.50
2.00
(B)
1.75
1.50
Final Volume, L
1.25
1.00
0.75
0.50-
0.25
0.00
0.75
1.00
0.00
0.25
Volume O₂ added, L
2
0.50
0.75
1.00
Volume O₂ added, L
2
2.00
2.00
(C)
(D)
1.75
1.75
1.50
1.50
Final Volume, L
1.25
1.00
0.75
0.50
Final Volume, L
1.25
1.00
0.75
0.50
0.25
0.25
0.00
0.00
0.00
0.25
0.50
0.75
1.00
0.00
0.25
Volume O₂ added, L
0.50
0.75
1.00
Volume O₂ added, L
2
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
Ch. 16.2 - Problem 17-1 Wrtie the IUPAC name for each...Ch. 16.2 - Prob. 16.2QCCh. 16.2 - Prob. 16.3QCCh. 16.4 - Prob. 16.4QCCh. 16.4 - Prob. 16.5QCCh. 16.4 - Problem 17-6 Show the reaction of benzaldehyde...Ch. 16.4 - Problem 17-7 Identify all hemiacetals and acetals...Ch. 16.5 - Prob. 16.8QCCh. 16 - 17-9 Answer true or false. (a) The one aldehyde...Ch. 16 - Prob. 2P
Ch. 16 - 17-11 What is the difference in structure between...Ch. 16 - 17-12 Is it possible for the carbon atom of a...Ch. 16 - 17-13 Which compounds contain carbonyl groups?Ch. 16 - 17-14 Following are structural formulas for two...Ch. 16 - 17-15 Draw structural formulas for the four...Ch. 16 - Prob. 8PCh. 16 - Prob. 9PCh. 16 - 17-18 Draw structural formulas for these ketones....Ch. 16 - 17-19 Write the JUPAC names for these compounds.Ch. 16 - Prob. 12PCh. 16 - 17-2 1 Explain why each name is incorrect. Write...Ch. 16 - Prob. 14PCh. 16 - Prob. 15PCh. 16 - 17-24 In each pair of compounds, select the one...Ch. 16 - Prob. 17PCh. 16 - 17-26 Account for the fact that acetone has a...Ch. 16 - 17-27 Pentane, 1-butanol, and butanal all have...Ch. 16 - 17-28 Show how acetaldehyde can form hydrogen...Ch. 16 - 17-29 Why can’t two molecules of acetone form a...Ch. 16 - 17-30 Answer true or false. (a) The reduction of...Ch. 16 - 17-3 1 Draw a structural formula for the principal...Ch. 16 - Prob. 24PCh. 16 - 17-33 What simple chemical test could you use to...Ch. 16 - 17-34 Explain why liquid aldehydes are often...Ch. 16 - 17-35 Suppose that you take a bottle of...Ch. 16 - 17-36 Explain why the reduction of an aldehyde...Ch. 16 - Prob. 29PCh. 16 - Prob. 30PCh. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Prob. 33PCh. 16 - Prob. 34PCh. 16 - Prob. 35PCh. 16 - Prob. 36PCh. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - 17-47 What is the characteristic structural...Ch. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - Prob. 43PCh. 16 - Prob. 44PCh. 16 - Prob. 45PCh. 16 - 17-54 Following is the structure of...Ch. 16 - Prob. 47PCh. 16 - Prob. 48PCh. 16 - Prob. 49PCh. 16 - Prob. 50PCh. 16 - Prob. 51PCh. 16 - 17-60 1-Propanol can be prepared by the reduction...Ch. 16 - Prob. 53PCh. 16 - 17-62 Show how to bring about these conversions....Ch. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - Prob. 57PCh. 16 - Prob. 58PCh. 16 - 17-67 Draw structural formulas for these...Ch. 16 - Prob. 60PCh. 16 - 17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C)...Ch. 16 - 17-70 What simple chemical test could you use to...Ch. 16 - Prob. 63PCh. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - 17-72 The following molecule is an enediol; each...Ch. 16 - 17-73 Alcohols can be prepared by the...Ch. 16 - 17-74 Glucose, C6H12O6, contains an aldehyde group...Ch. 16 - Prob. 69PCh. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - 17-78 Complete the following equation for these...Ch. 16 - 17-79 Write an equation for each conversion. (a)...Ch. 16 - Prob. 74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Leucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forwarda) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward12. CH3 OH OH H&C CH3 H₂C N OH H₂C CH3 H&C CH3 H₂C' CH3 H.C CH3OH H.C CH2CH3OH CH3CEN Which one of these 17 compounds is represented by this IR and this 'H NMR spectrum? IR Spectrum 3000 4000 3000 NMR Spectrum 2000 £500 RAVENUMBER 2000 1500 9 8 6 5 10 HP-00-290 ppm m 1000 500 1000 4 °arrow_forward
- Draw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forward
- Write "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forward
- Do you do chemistry assignmentsarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A This reaction is always spontaneous, but proceeds slower at temperatures above 120. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 117. °C. AS is (pick one) ΔΗ is (pick one) This reaction is slower below 20. °C than C above. AS is |(pick one) ? 18 Ar 1arrow_forwardCalculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Molecular spectroscopy; Author: Vidya-mitra;https://www.youtube.com/watch?v=G6HjLIWvCQo;License: Standard YouTube License, CC-BY