
Concept explainers
(a)
Interpretation:
The structural formula of keto form for the given enol form should be drawn.
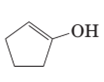
Concept Introduction:
A carbonyl compound that has a-hydrogen on a-carbon changes its form to enol through resonance. Both the form stays in equilibrium.
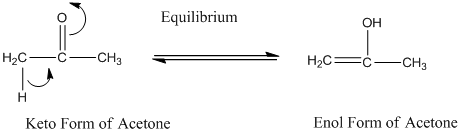
a- hydrogen is the hydrogen which attached to a-carbon for a carbonyl compound. a-carbon is carbon adjacent to the carbonyl carbon. If a compound don’t have any a hydrogen is does not undergoes Keto-Enol tautomerism.
(b)
Interpretation:
The structural formula of keto form for the given enol form should be drawn.
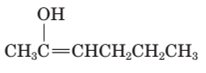
Concept Introduction:
A carbonyl compound that has a-hydrogen on a-carbon changes its form to enol through resonance. Both the form stays in equilibrium.
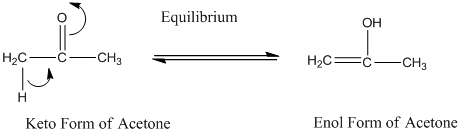
a- hydrogen is the hydrogen which attached to a-carbon for a carbonyl compound. a-carbon is carbon adjacent to the carbonyl carbon. If a compound don’t have any a hydrogen is does not undergoes Keto-Enol tautomerism.
(c)
Interpretation:
The structural formula of keto form for the given enol form should be drawn.
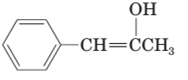
Concept Introduction:
A carbonyl compound that has a-hydrogen on a-carbon changes its form to enol through resonance. Both the form stays in equilibrium.
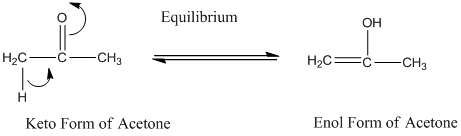
a- hydrogen is the hydrogen which attached to a a-carbon for a carbonyl compound. a-carbon is carbon adjacent to the carbonyl carbon. If a compound don’t have any a hydrogen is does not undergoes Keto-Enol tautomerism.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- A chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forward
- To improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forwardplease draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardNaming and drawing secondary Write the systematic (IUPAC) name for each of the following organic molecules: CH3 Z structure CH3 CH2 CH2 N-CH3 CH3-CH2-CH2-CH-CH3 NH CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3 Explanation Check ☐ name ☐ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C Garrow_forward
- C This question shows how molecular orbital (MO) theory can be used to understand the chemical properties of elemental oxygen O₂ and its anionic derivative superoxide Oz. a) Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names and molecular orbital symmetry labels and include electrons. Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and how does this difference relate to the high reactivity and magnetic properties of oxygen? ) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).arrow_forwardPlease drawarrow_forward-Page: 8 nsition metal ions have high-spin aqua complexes except one: [Co(HO)₁]". What is the d-configuration, oxidation state of the metal in [Co(H:O))"? Name and draw the geometry of [Co(H2O)]? b) Draw energy diagrams showing the splitting of the five d orbitals of Co for the two possible electron configurations of [Co(H2O)]: Knowing that A = 16 750 cm and Пl. = 21 000 cm, calculate the configuration energy (.e., balance or ligand-field stabilization energy and pairing energy) for both low spin and high spin configurations of [Co(H2O)]. Which configuration seems more stable at this point of the analysis? (Note that 349.76 cm = 1 kJ/mol) Exchange energy (IT) was not taken into account in part (d), but it plays a role. Assuming exchange an occur within t29 and within eg (but not between tz, and ea), how many exchanges are possible in the low in configuration vs in the high spin configuration? What can you say about the importance of exchange energy 07arrow_forward
- Draw everything please on a piece of paper explaining each steparrow_forwardDefine crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





