
(a)
Interpretation:
The structure of six-membered cyclic hemiacetal formed by 5-hydroxyhexanal should be drawn.
Concept Introduction:
Hemiacetal is a molecule formed by the addition of an alcohol to an
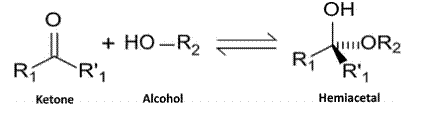
Answer to Problem 63P
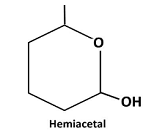
Explanation of Solution
Reaction of the aldehyde group and the hydroxyl group forms a six-membered cyclic hemiacetal.
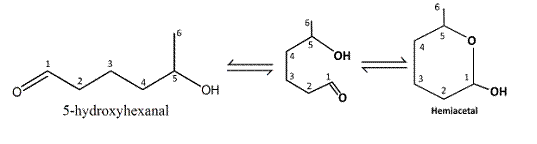
Therefore, the structure hemiacetal formed from 5-hydroxyhexanalis as follows:
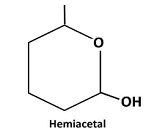
(b)
Interpretation:
The number of possible stereoisomers of 5-hydroxyhexanal should be determined.
Concept Introduction:
Stereoisomers are the compounds that are differ only in the spatial arrangements of their atoms. For a molecule with n stereoisomers, a maximum of
Answer to Problem 63P
5-hydroxyhexanal has 2 stereoisomers.
Explanation of Solution
For a molecule with n stereoisomers, a maximum of
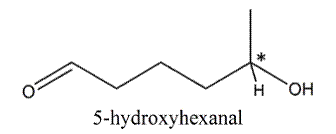
In 5-hydroxyhexanal, there is one stereocenter.
Therefore, number of stereoisomers can be calculated as follows:
Thus, number of stereoisomers will be 2.
(c)
Interpretation:
The number of possible stereoisomers of hemiacetal of 5-hydroxyhexanal should be calculated.
Concept Introduction:
Stereoisomers are the compounds that are differ only in the spatial arrangements of their atoms. For a molecule with n stereoisomers, a maximum of
Answer to Problem 63P
Hemiacetal of 5-hydroxyhexanal has 4 stereoisomers.
Explanation of Solution
For a molecule with n stereoisomers, a maximum of
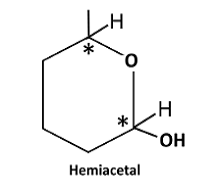
In hemiacetal of 5-hydroxyhexanal, there are two stereo centres.
Therefore, number of stereoisomers can be calculated as follows:
Thus, number of stereoisomers are 4.
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


