
(a)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Answer to Problem 57P
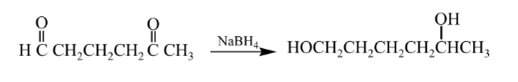
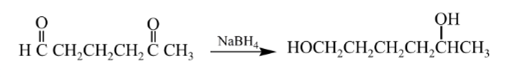
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
(b)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Answer to Problem 57P
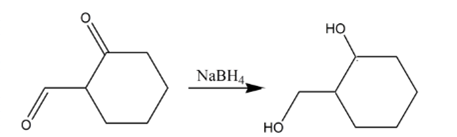
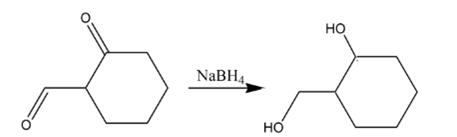
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
(c)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Answer to Problem 57P
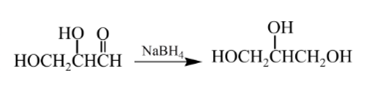
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
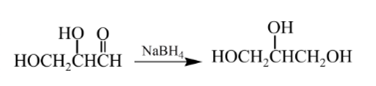
(d)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Answer to Problem 57P
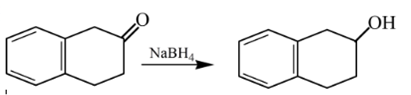
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
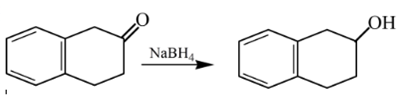
(e)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
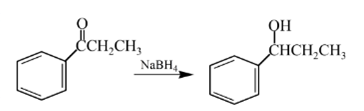
Answer to Problem 57P
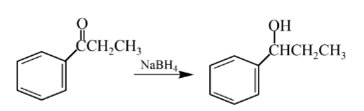
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
(f)
Interpretation:
Draw the structural formula the compound formed by the reaction of given compound with sodium borohydride.
Concept Introduction:
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
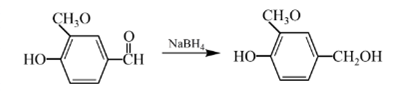
Answer to Problem 57P
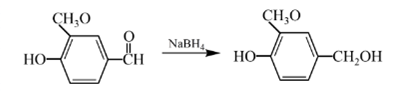
Explanation of Solution
Aldehydes are reduced to primary alcohols and ketones are reduced in secondary alcohols. The most commonly used reagent for reduction of aldehydes and ketone is sodium borohydride
Therefore, the product formed will be as follows:
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- HOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardDraw the mechanism for the oxidation of 3-bromo-cyclohexan-1-ol.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


