
(a)
Interpretation:
The structural formula for the principal organic product formed when Butanal is reacted with.
Tollen’s reagent should be drawn.
Concept Introduction:
Tollen’s regent is reacts with the
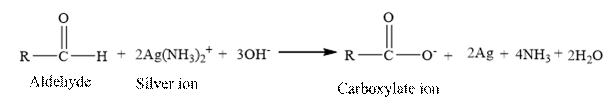
Butanal is an aldehyde. The structure of Butanal is as follows.
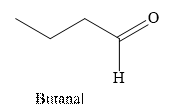
(b)
Interpretation:
The structural formula for the principal organic product formed when Benzaldehyde is reacted with Tollen’s reagent should be drawn.
Concept Introduction:
Tollen’s regent is reacts with the aldehyde group of a compound. The carbonyl group acts as a reducing agent and reduces the silver ion of the Tollen’s regent to silver metal.
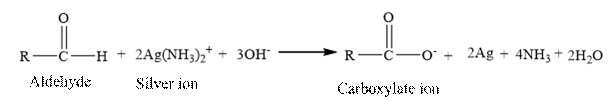
Benzaldehyde is an aldehyde. The structure of benzaldehyde is as follows.
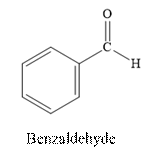
(c)
Interpretation:
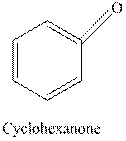
The structural formula for the principal organic product formed when cyclohexanone is reacted with Tollen’s reagent should be drawn.
Concept Introduction:
Tollen’s regent is reacts with the aldehyde group of a compound. The carbonyl group acts as a reducing agent and reduces the silver ion of the Tollen’s regent to silver metal.
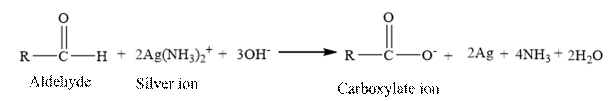
Cyclohexanone is a
(d)
Interpretation:
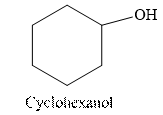
The structural formula for the principal organic product formed when cyclohexanol is treated with Tollen’s reagent.
Concept Introduction:
Tollen’s regent is reacts with the aldehyde group of a compound. The carbonyl group acts as a reducing agent and reduces the silver ion of the Tollen’s regent to silver metal.
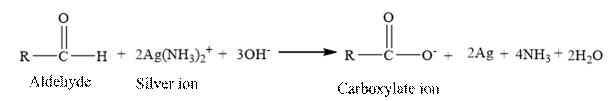
Cyclohexanol is an alcohol. The structure of cyclohexanol is as follows.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
- Feedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning




