
Concept explainers
17-9 Answer true or false.
(a) The one aldehyde and the one ketone with a molecular formula of C3H6O are constitutional isomers.
(b)
(c) The VSEPR model predicts bond angles of 1200 about the carbonyl carbon of aldehydes and ketones.
(d) The carbonyl carbon of a ketone is a stereocenter.
(a)
Interpretation:
Answer true or false for the following statement.
The one aldehyde and one ketone with a molecular formula of
Concept Introduction:
Constitutional isomers have the same molecular formula but different structural formulas or connectivity with atoms is different. In aldehydes, the carbonyl group is bonded to a hydrogen atom and ketones carbonyl group is bonded to two carbon atoms.
Answer to Problem 1P
The one aldehyde and one ketone with a molecular formula of
Explanation of Solution
The given molecular formula:
For this molecular formula we can draw both aldehyde and ketone. They are as follows.
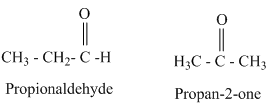
From the above structures aldehydes and ketones having same molecular formula are constitutional isomers.
Therefore, the given statement is true.
(b)
Interpretation:
Answer true or false for the following statement.
Aldehydes and ketones both contain a carbonyl group.
Concept Introduction:
In aldehydes, the carbonyl group is bonded to a hydrogen atom and ketones carbonyl group is bonded to two carbon atoms.
Answer to Problem 1P
Aldehydes and ketones both contain a carbonyl group is true statement.
Explanation of Solution
In aldehydes, the carbonyl group is bonded to a hydrogen atom and in ketone carbonyl group is bonded to two carbon atoms. Hence, both an aldehyde and a ketone have a carbonyl group.

Where, R is an alkyl group.
Therefore, the given statement is true.
(c)
Interpretation:
Answer true or false for the following statement.
The VSEPR model predicts bond angles of 1200 about the carbonyl carbon of aldehydes and ketones.
Concept Introduction:
In aldehydes, the carbonyl group is bonded to a hydrogen atom and ketones carbonyl group is bonded to two carbon atoms. VSEPR model predicts the geometry of the molecule with the help of number of pair of electrons present around the central atoms and bond angle of the molecule.
Answer to Problem 1P
The VSEPR model predicts bond angles of 1200 about the carbonyl carbon if aldehydes and ketones is the true statement.
Explanation of Solution
In aldehydes, the carbonyl group is bonded to a hydrogen atom and ketones carbonyl group is bonded to two carbon atoms. The carbonyl group of aldehyde and ketone are represented are as follows.
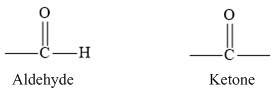
According to VSEPR theory, three pair of electron groups forms a Trigonal planar geometry with bond angle of 1200.
Therefore, the given statement is true.
(d)
Interpretation:
Answer true or false for the following statement.
The carbonyl carbon of ketone is a stereocentre.
Concept Introduction:
A stereocentre is a tetrahedral carbon atom in which four different groups are attached to it. When an atom generally carbon is linked with four different groups, then that centre is known as chiral centre.
Answer to Problem 1P
The carbonyl carbon of ketone is a stereocenter is the false statement.
Explanation of Solution
In aldehydes, the carbonyl group is bonded to a hydrogen atom and in ketone carbonyl group is bonded to two carbon atoms. The carbonyl group of aldehyde and ketone are represented are as follows.

In ketones, the carbonyl carbon is boded to carbonyl oxygen by a double bond group and bonded to two other carbon atoms.
Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- Problem Set 4a Chem 1411. A latex balloon is filled with a total of carbon dioxide gas so that its volume reaches 1.352 L. The balloon whose weight was originally 0.753 g, now weighs 2.538 g. How many molecules of carbon dioxide have been added to the balloon?arrow_forwardQ18. 30 minutes left please help!!arrow_forwardQ35. Please help wth these drawings! I only have an hour left!!arrow_forward
- Briefly indicate and with examples the differences between metallic cluster and cage compound.arrow_forwardIndicate the correct answer.a) In boranes, the B-B bonds are the most reactive.b) The B-H-B bonds are the reactive centers in the B2H6 molecule.arrow_forwardIn boranes, the B-B bonds are the most reactive.arrow_forward
- The B-H-B bonds are the reactive centers in the B2H6 molecule. Correct?arrow_forwardPlease help me choose! {Apparently B is wrong}arrow_forward13) Which of the following configurations corresponds to the structure below? С соон SH Br 8H H CHBrCH3 a) (2S, 3S) (2S, 3R) c) (2R,3S) d) (2R, 3R)arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




