
Essential University Physics Volume 1, Loose Leaf Edition (4th Edition)
4th Edition
ISBN: 9780135264669
Author: Richard Wolfson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 46P
A circular lake 1.0 km in diameter is 10 m deep (Fig. 16.14). Solar energy is incident on the lake at an average rate of 200 W/m2. If the lake absorbs all this energy and does not exchange heat with its surroundings, how long will it take to warm from 10°C to 20°C?
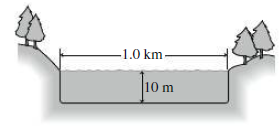
FIGURE 16.14 Problem 46
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
It is not possible to see very small objects, such as viruses, using an ordinary light microscope. An electron microscope can view such objects using an electron beam instead of a light beam. Electron microscopy has proved invaluable for investigations of viruses, cell membranes and subcellular structures, bacterial surfaces, visual receptors, chloroplasts, and the contractile properties of muscles. The "lenses" of an
electron microscope consist of electric and magnetic fields that control the electron beam.
As an example of the manipulation of an electron beam, consider an electron traveling away from the origin along the x axis in the xy plane with initial velocity ₁ = vi. As it passes through the region x = 0 to x=d, the electron experiences acceleration a = ai +a, where a and a, are constants. For the case v, = 1.67 x 107 m/s, ax = 8.51 x 1014 m/s², and a = 1.50 x 10¹5 m/s², determine the following at
x = d = 0.0100 m.
(a) the position of the electron
y, = 2.60e1014
m
(b) the…
No chatgpt pls
need help with the first part
Chapter 16 Solutions
Essential University Physics Volume 1, Loose Leaf Edition (4th Edition)
Ch. 16.1 - Is there (a) no temperature, (b) one temperature,...Ch. 16.2 - A hot rock with mass 250 g is dropped into an...Ch. 16.3 - The figure shows three slabs with the same...Ch. 16.3 - Prob. 16.4GICh. 16.4 - A houses thermostat fails, leaving the furnace...Ch. 16 - If system A is not in thermodynamic equilibrium...Ch. 16 - Does a thermometer measure its own temperature or...Ch. 16 - Compare the relative sizes of the kelvin, the...Ch. 16 - If you put a thermometer in direct sunlight, what...Ch. 16 - Why does the temperature in a stone building...
Ch. 16 - Why do large bodies of water exert a...Ch. 16 - A Thermos bottle consists of an evacuated,...Ch. 16 - Stainless-steel cookware often has a layer of...Ch. 16 - Prob. 9FTDCh. 16 - Prob. 10FTDCh. 16 - Glass and fiberglass are made from the same...Ch. 16 - To keep your hands warm while skiing, you should...Ch. 16 - Since Earth is exposed to solar radiation, why...Ch. 16 - Global warming at Earths surface is generally...Ch. 16 - In its 2014 report, the Intergovernmental Panel on...Ch. 16 - A Canadian meteorologist predicts an overnight low...Ch. 16 - Normal room temperature is 68F. Whats this in...Ch. 16 - Prob. 18ECh. 16 - At what temperature do the Fahrenheit and Celsius...Ch. 16 - The normal boiling point of nitrogen is 77.3 K....Ch. 16 - Prob. 21ECh. 16 - Prob. 22ECh. 16 - Prob. 23ECh. 16 - Whats the specific heat of a material if it takes...Ch. 16 - The average human diet contains about 2000 kcal...Ch. 16 - Prob. 26ECh. 16 - You bring a 350-g wrench into the house from your...Ch. 16 - Prob. 28ECh. 16 - Building heat loss in the United States is usually...Ch. 16 - Find the heat-loss rate through a slab of (a) wood...Ch. 16 - The top of a steel wood stove measures 90 cm by 40...Ch. 16 - Youre a builder whos advising a homeowner to have...Ch. 16 - An 8.0 m by 12 m house is built on a concrete slab...Ch. 16 - Find the -factor for a wall that loses 0.040 Btu...Ch. 16 - Compute the -factors for 1-inch thicknesses of...Ch. 16 - A horseshoe has surface area 50 cm2, and a...Ch. 16 - An oven loses energy at the rate of 14 W per C...Ch. 16 - Youre having your homes heating system replaced,...Ch. 16 - The filament of a 100-W lightbulb is at 3.0 kK....Ch. 16 - A typical human body has surface area 1.4 nr and...Ch. 16 - A constant-volume gas thermometer is filled with...Ch. 16 - A constant-volume gas thermometer is at 55-kPa...Ch. 16 - In Fig. 16.2s gas thermometer, the height h is...Ch. 16 - Prob. 44PCh. 16 - Typical fats contain about 9 kcal per gram. If the...Ch. 16 - A circular lake 1.0 km in diameter is 10 m deep...Ch. 16 - How much heat is required to raise an 800-g copper...Ch. 16 - Initially, 100 g of water and 100 g of another...Ch. 16 - Prob. 49PCh. 16 - Two neighbors return from Florida to find their...Ch. 16 - Prob. 51PCh. 16 - Prob. 52PCh. 16 - Prob. 53PCh. 16 - The temperature of the eardrum provides a reliable...Ch. 16 - Prob. 55PCh. 16 - Your young niece complains that her cocoa, at 90C,...Ch. 16 - A piece of copper at 300C is dropped into 1.0 kg...Ch. 16 - While camping, you boil water to make spaghetti....Ch. 16 - A biology labs walk-in cooler measures 3.0 m by...Ch. 16 - One end of an iron rod 40 cm long and 3.0 cm in...Ch. 16 - Prob. 61PCh. 16 - An electric stove burner has surface area 325 cm2...Ch. 16 - An electric current passes through a metal strip...Ch. 16 - Youre considering purchasing a new sleeping bag...Ch. 16 - A blacksmith heats a 1.1-kg iron horseshoe to...Ch. 16 - Whats the power output of a microwave oven that...Ch. 16 - A cylindrical log 15 cm in diameter and 65 cm long...Ch. 16 - A blue giant star whose surface temperature is 23...Ch. 16 - Prob. 69PCh. 16 - A black wood stove with surface area 4.6 nr is...Ch. 16 - Estimate the average temperature on Pluto,...Ch. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - In a cylindrical pipe where area isnt constant....Ch. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Prob. 79PCh. 16 - Use the method outlined in Problem 76 to show that...Ch. 16 - A house is at 20C on a winter night when the...Ch. 16 - A more realistic approach to the solar greenhouse...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
What properties do all types of epithelia share?
Campbell Biology (11th Edition)
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautome...
Organic Chemistry (8th Edition)
In a population, what is the consequence of inbreeding? Does inbreeding change allele frequencies? What is the ...
Genetic Analysis: An Integrated Approach (3rd Edition)
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A ball is thrown with an initial speed v, at an angle 6, with the horizontal. The horizontal range of the ball is R, and the ball reaches a maximum height R/4. In terms of R and g, find the following. (a) the time interval during which the ball is in motion 2R (b) the ball's speed at the peak of its path v= Rg 2 √ sin 26, V 3 (c) the initial vertical component of its velocity Rg sin ei sin 20 (d) its initial speed Rg √ sin 20 × (e) the angle 6, expressed in terms of arctan of a fraction. 1 (f) Suppose the ball is thrown at the same initial speed found in (d) but at the angle appropriate for reaching the greatest height that it can. Find this height. hmax R2 (g) Suppose the ball is thrown at the same initial speed but at the angle for greatest possible range. Find this maximum horizontal range. Xmax R√3 2arrow_forwardAn outfielder throws a baseball to his catcher in an attempt to throw out a runner at home plate. The ball bounces once before reaching the catcher. Assume the angle at which the bounced ball leaves the ground is the same as the angle at which the outfielder threw it as shown in the figure, but that the ball's speed after the bounce is one-half of what it was before the bounce. 8 (a) Assuming the ball is always thrown with the same initial speed, at what angle & should the fielder throw the ball to make it go the same distance D with one bounce (blue path) as a ball thrown upward at 35.0° with no bounce (green path)? 24 (b) Determine the ratio of the time interval for the one-bounce throw to the flight time for the no-bounce throw. Cone-bounce no-bounce 0.940arrow_forwardA rocket is launched at an angle of 60.0° above the horizontal with an initial speed of 97 m/s. The rocket moves for 3.00 s along its initial line of motion with an acceleration of 28.0 m/s². At this time, its engines fail and the rocket proceeds to move as a projectile. (a) Find the maximum altitude reached by the rocket. 1445.46 Your response differs from the correct answer by more than 10%. Double check your calculations. m (b) Find its total time of flight. 36.16 x Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate results to at least four-digit accuracy to minimize roundoff error. s (c) Find its horizontal range. 1753.12 × Your response differs from the correct answer by more than 10%. Double check your calculations. marrow_forward
- Race car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? Please answer parts a-B. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places. DONT FORGET TO DRAW VECTORS! ONLY USE BASIC FORMULAS TAUGHT IN PHYSICS. distance = speed * time.arrow_forwardRace car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? c) If the driver’s average rate of acceleration is -9.5 m/s2 as he slows down, how long does it take him to come to a stop (use information about his speed of 28.9 m/s but do NOT use his reaction and movement time in this computation)? Please answer parts a-c. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise.…arrow_forwardHow is it that part a is connected to part b? I can't seem to solve either part and don't see the connection between the two.arrow_forward
- Hello, please help with inputing trial one into the equation, I just need a model for the first one so I can answer the rest. Also, does my data have the correct sigfig? Thanks!arrow_forwardFind the current in the R₁ resistor in the drawing (V₁=16.0V, V2=23.0 V, V₂ = 16.0V, R₁ = 2005, R₂ = and R₂ = 2.705) 2.3052 VIT A www R www R₂ R₂ Vaarrow_forwardWhich of the following laws is true regarding tensile strength? • tensile strength T ①Fbreak = Wtfest Piece thickness rate (mm) ②T = test piece width rabe (mm) Fbreak break watarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY