1 Doing Physics 2 Motion In A Straight Line 3 Motion In Two And Three Dimensions 4 Force And Motion 5 Using Newton's Laws 6 Energy, Work, And Power 7 Conservation Of Energy 8 Gravity 9 Systems Of Particles 10 Rotational Motion 11 Rotational Vectors And Angular Momentum 12 Static Equilibrium 13 Oscillatory Motion 14 Wave Motion 15 Fluid Motion 16 Temperature And Heat 17 The Thermal Behavior Of Matter 18 Heat, Work, And The First Law Of Thermodynamics 19 The Second Law Of Thermodynamics expand_more
16.1 Heat, Temperature, And Thermodynamic Equilibrium 16.2 Heat Capacity And Specific Heat 16.3 Heat Transfer 16.4 Themal-Energy Balance Chapter Questions expand_more
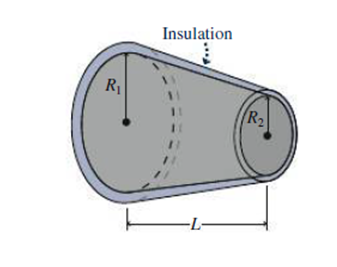
Problem 1FTD: If system A is not in thermodynamic equilibrium with system B, and B is not in equilibrium with C,... Problem 2FTD: Does a thermometer measure its own temperature or the temperature of its surroundings? Explain. Problem 3FTD: Compare the relative sizes of the kelvin, the degree Celsius, the degree Fahrenheit, and the degree... Problem 4FTD: If you put a thermometer in direct sunlight, what do you measure: the air temperature, the... Problem 5FTD: Why does the temperature in a stone building usually vary less than in a wooden building? Problem 6FTD: Why do large bodies of water exert a temperature-moderating effect on their surroundings? Problem 7FTD: A Thermos bottle consists of an evacuated, double-wall glass liner, coated with a thin layer of... Problem 8FTD: Stainless-steel cookware often has a layer of aluminum or copper embedded in the bottom. Why? Problem 9FTD Problem 10FTD Problem 11FTD: Glass and fiberglass are made from the same material, yet have dramatically different thermal... Problem 12FTD: To keep your hands warm while skiing, you should wear mittens instead of gloves. Why? Problem 13FTD: Since Earth is exposed to solar radiation, why doesnt Earth have the same temperature as the Sun? Problem 14FTD: Global warming at Earths surface is generally producing greater temperature rises over land than... Problem 15E: In its 2014 report, the Intergovernmental Panel on Climate Change projected a global temperature... Problem 16E: A Canadian meteorologist predicts an overnight low of 15C. How would a U.S. meteorologist express... Problem 17E: Normal room temperature is 68F. Whats this in Celsius? Problem 18E Problem 19E: At what temperature do the Fahrenheit and Celsius scales coincide? Problem 20E: The normal boiling point of nitrogen is 77.3 K. Express this in Celsius and Fahrenheit. Problem 21E Problem 22E Problem 23E Problem 24E: Whats the specific heat of a material if it takes 7.5 kJ to increase the temperature of a 1-kg... Problem 25E: The average human diet contains about 2000 kcal per day. If all this food energy is released rather... Problem 26E Problem 27E: You bring a 350-g wrench into the house from your car. The house is 15C warmer than the car, and it... Problem 28E Problem 29E: Building heat loss in the United States is usually expressed in Btu/h. Whats 1 Btu/h in SI units? Problem 30E: Find the heat-loss rate through a slab of (a) wood and (b) Styrofoam, each 2.0 cm thick, if one... Problem 31E: The top of a steel wood stove measures 90 cm by 40 cm and is 0.45 cm thick. The fire maintains the... Problem 32E: Youre a builder whos advising a homeowner to have her foundation walls insulated with 2 inches of... Problem 33E: An 8.0 m by 12 m house is built on a concrete slab 23 cm thick. Find the heat-loss rate through the... Problem 34E: Find the -factor for a wall that loses 0.040 Btu each hour through each square foot for each F... Problem 35E: Compute the -factors for 1-inch thicknesses of air, concrete, fiberglass, glass, Styrofoam, and... Problem 36E: A horseshoe has surface area 50 cm2, and a blacksmith heats it to a red-hot 810C. At what rate does... Problem 37E: An oven loses energy at the rate of 14 W per C temperature difference between its interior and the... Problem 38E: Youre having your homes heating system replaced, and the heating contractor has specified a new... Problem 39E: The filament of a 100-W lightbulb is at 3.0 kK. Whats the filaments surface area? Problem 40E: A typical human body has surface area 1.4 nr and skin temperature 33C. If the bodys emissivity is... Problem 41P: A constant-volume gas thermometer is filled with air whose pressure is 101 kPa at the normal melting... Problem 42P: A constant-volume gas thermometer is at 55-kPa pressure at the triple point of water. By how much... Problem 43P: In Fig. 16.2s gas thermometer, the height h is 60.0 mm at the triple point of water. When the... Problem 44P Problem 45P: Typical fats contain about 9 kcal per gram. If the energy in body fat could be utilized with 100%... Problem 46P: A circular lake 1.0 km in diameter is 10 m deep (Fig. 16.14). Solar energy is incident on the lake... Problem 47P: How much heat is required to raise an 800-g copper pan from 15C to 90C if (a) the pan is empty or... Problem 48P: Initially, 100 g of water and 100 g of another substance listed in Table 16.1 are at 20C. Heat is... Problem 49P Problem 50P: Two neighbors return from Florida to find their houses at a frigid 35F. Each house has a furnace... Problem 51P Problem 52P Problem 53P Problem 54P: The temperature of the eardrum provides a reliable measure of deep body temperature and is measured... Problem 55P Problem 56P: Your young niece complains that her cocoa, at 90C, is too hot. You pour 2 oz of milk at 3C into the... Problem 57P: A piece of copper at 300C is dropped into 1.0 kg of water at 20C. If the equilibrium temperature is... Problem 58P: While camping, you boil water to make spaghetti. Your pot contains 2.5 kg of water initially at 10C.... Problem 59P: A biology labs walk-in cooler measures 3.0 m by 2.0 m by 2.3 m and is insulated with 8.0-cm-thick... Problem 60P: One end of an iron rod 40 cm long and 3.0 cm in diameter is in ice water, the other in boiling water... Problem 61P Problem 62P: An electric stove burner has surface area 325 cm2 and emissivity e = 1. The burner consumes 1500 W... Problem 63P: An electric current passes through a metal strip 0.50 cm by 5.0 cm by 0.10 mm, heating it at a rate... Problem 64P: Youre considering purchasing a new sleeping bag whose manufacturer claims will keep you warm to 10F.... Problem 65P: A blacksmith heats a 1.1-kg iron horseshoe to 550C, then plunges it into a bucket containing 15 kg... Problem 66P: Whats the power output of a microwave oven that can heat 430 g of water from 20C to the boiling... Problem 67P: A cylindrical log 15 cm in diameter and 65 cm long is glowing red hot in a fireplace. The logs... Problem 68P: A blue giant star whose surface temperature is 23 kK radiates energy at the rate of 3.4 1030 W.... Problem 69P Problem 70P: A black wood stove with surface area 4.6 nr is made from cast iron 4.0 mm thick. Its interior wall... Problem 71P: Estimate the average temperature on Pluto, treating the dwarf planet as a blackbody whose great... Problem 72P Problem 73P Problem 74P Problem 75P Problem 76P: In a cylindrical pipe where area isnt constant. Equation 16.5 takes the form H = kA(dT/dr), where r... Problem 77P Problem 78P Problem 79P Problem 80P: Use the method outlined in Problem 76 to show that the steady heat-flow rate in the direction of the... Problem 81P: A house is at 20C on a winter night when the outside temperature is a steady 15C. The houses heat... Problem 82P: A more realistic approach to the solar greenhouse of Example 16.7 considers the time dependence of... Problem 83PP: Fiberglass is a popular, economical, and fairly effective building insulation. It consists of fine... Problem 84PP: Fiberglass is a popular, economical, and fairly effective building insulation. It consists of fine... Problem 85PP: Fiberglass is a popular, economical, and fairly effective building insulation. It consists of fine... Problem 86PP: Fiberglass is a popular, economical, and fairly effective building insulation. It consists of fine... format_list_bulleted


 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University




