
Concept explainers
Draw structural formulas for (1) the alkyltriphenylphosphonium salt formed by treatment of each
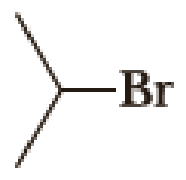
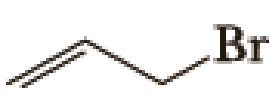
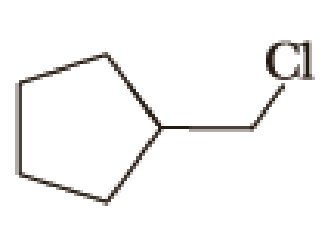
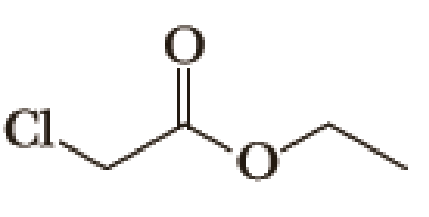

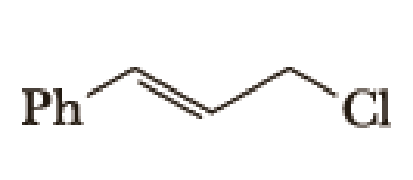
(a)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
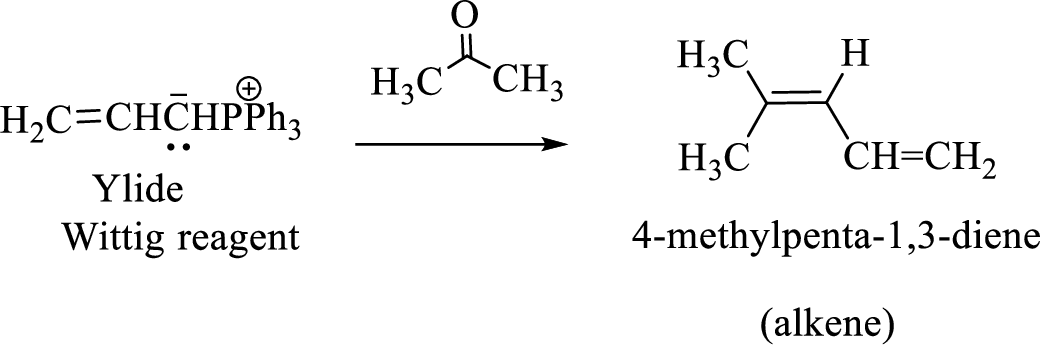
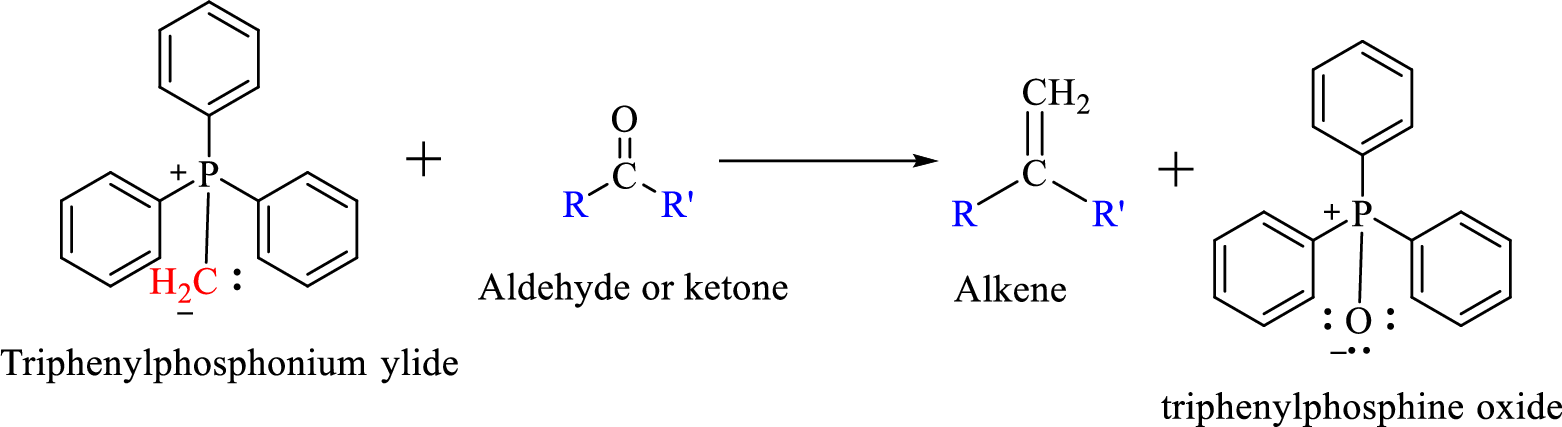
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
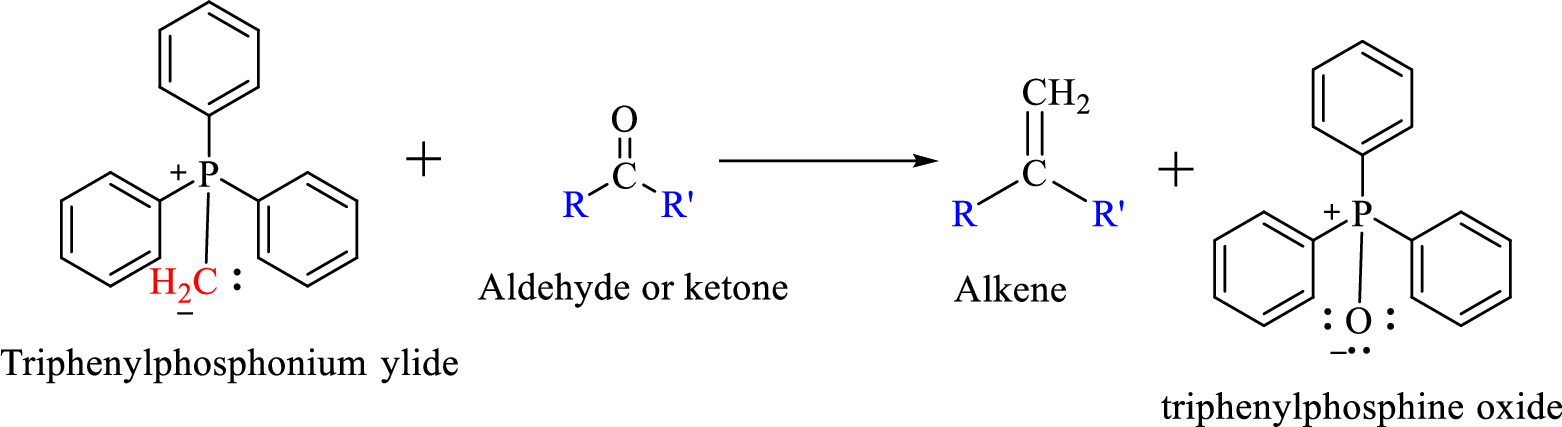
Mechanism:
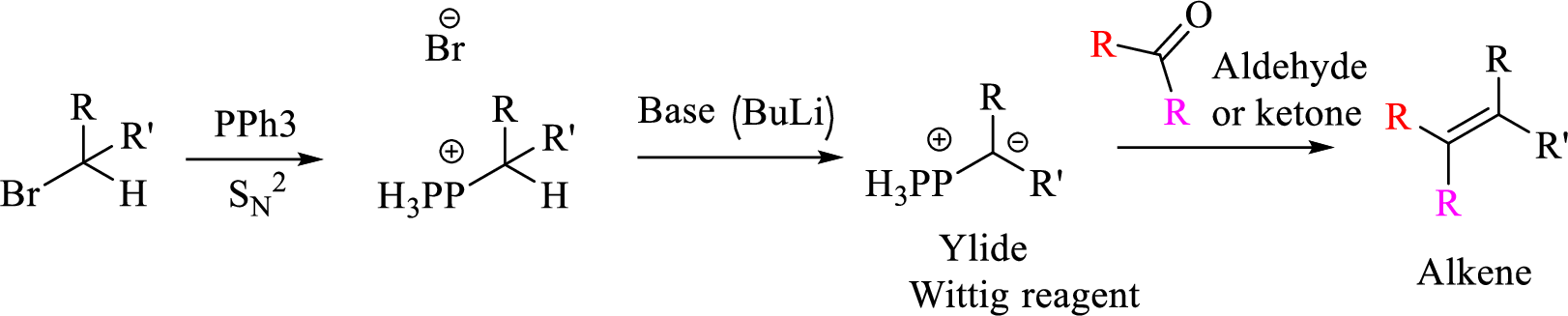
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
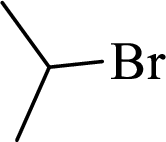
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
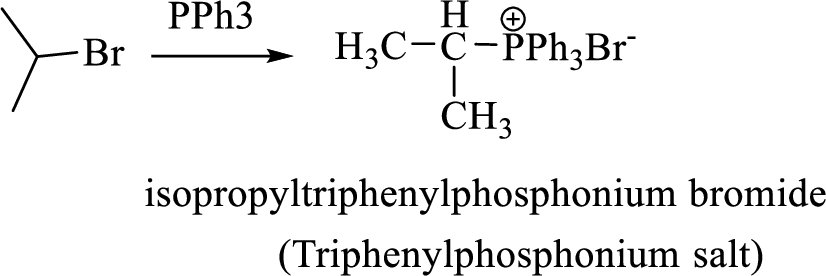
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
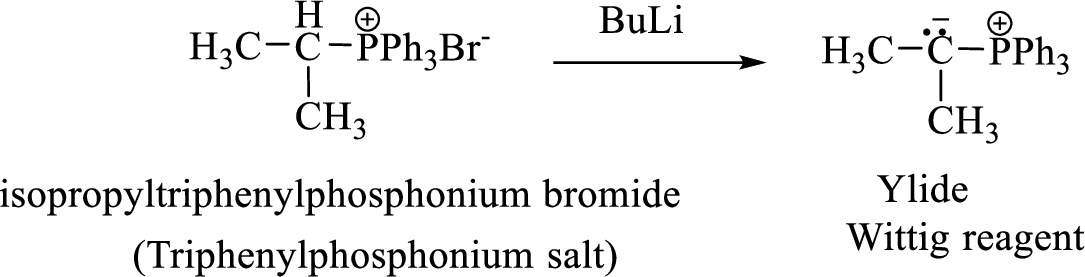
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
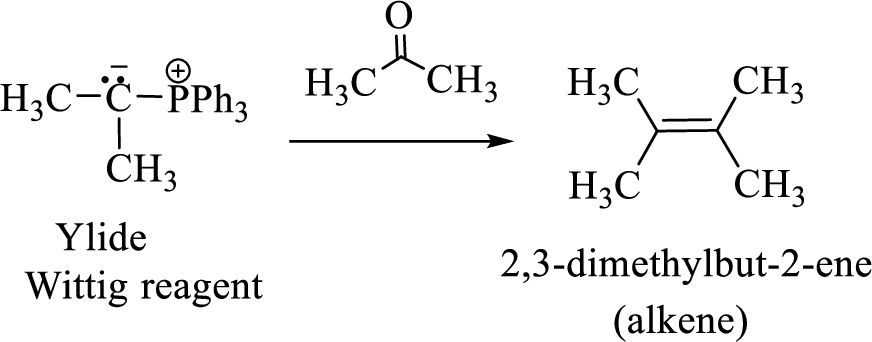
(b)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
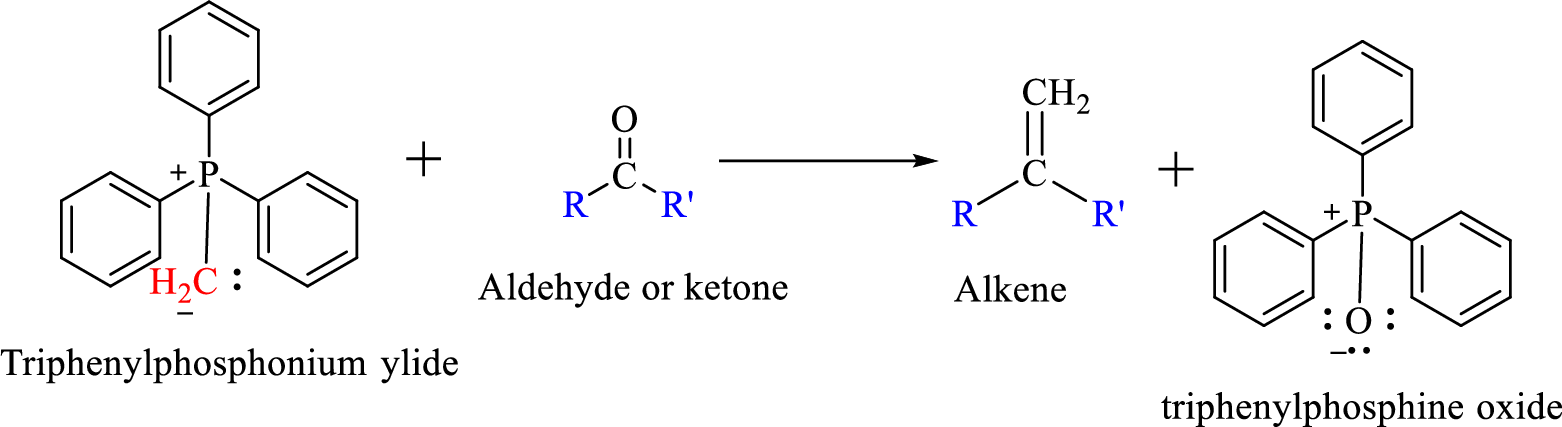
Mechanism:
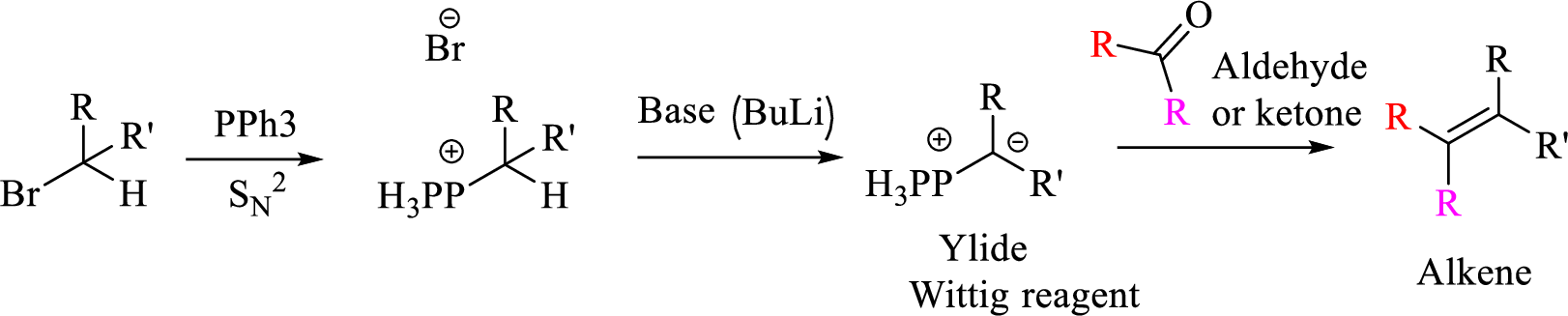
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
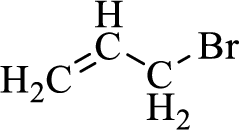
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
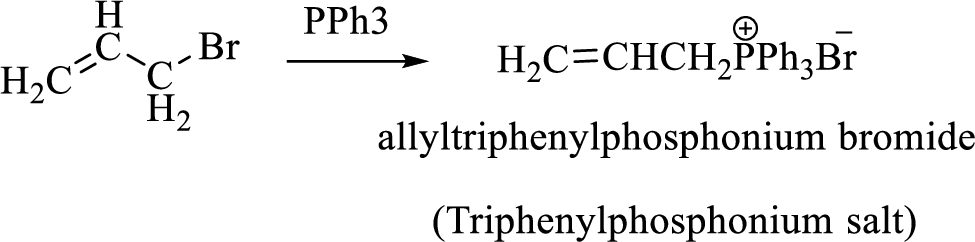
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
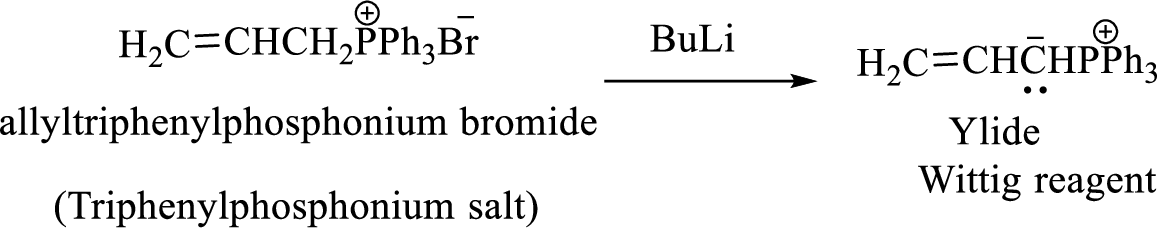
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
(c)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
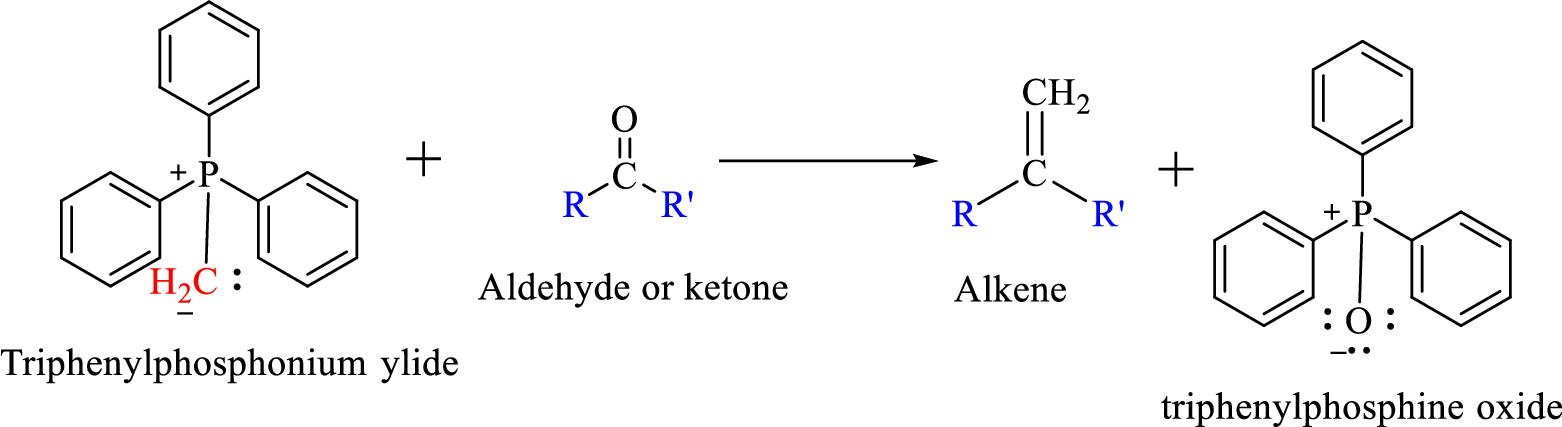
Mechanism:
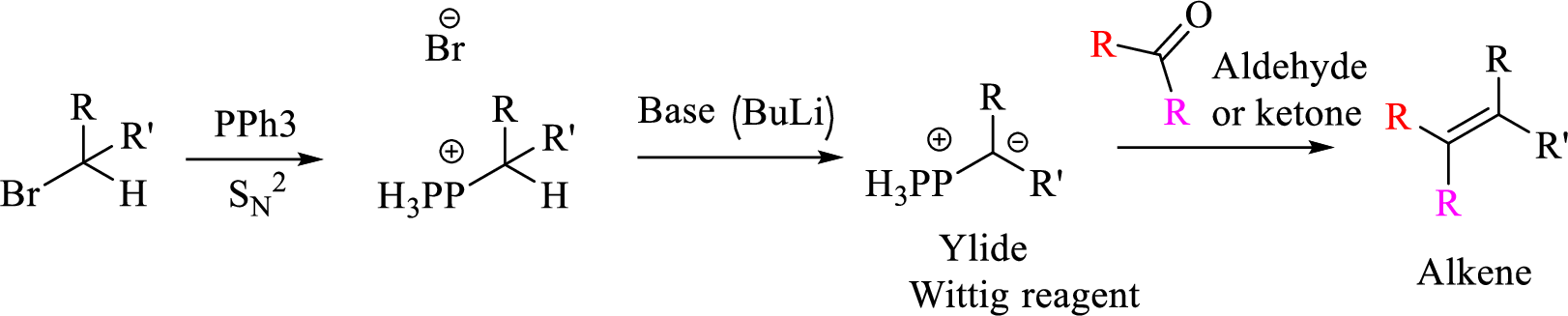
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
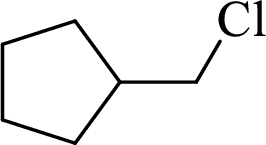
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
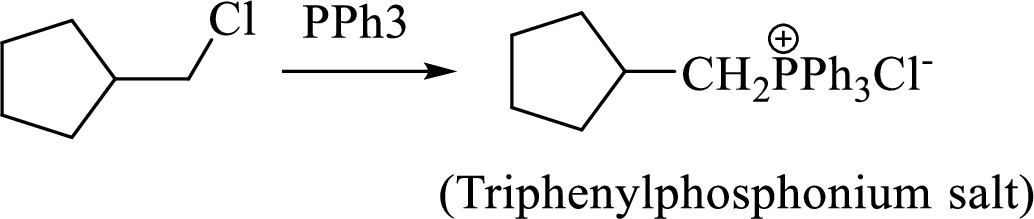
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
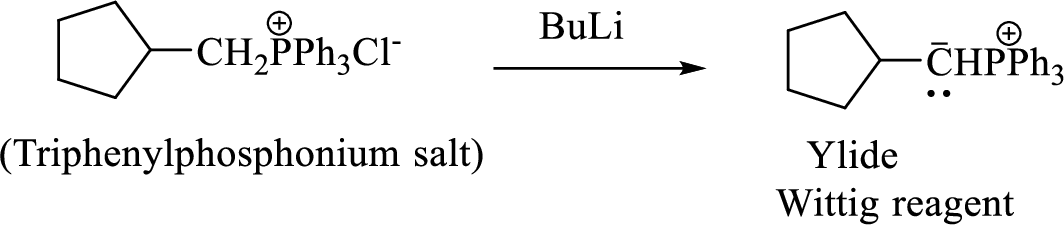
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
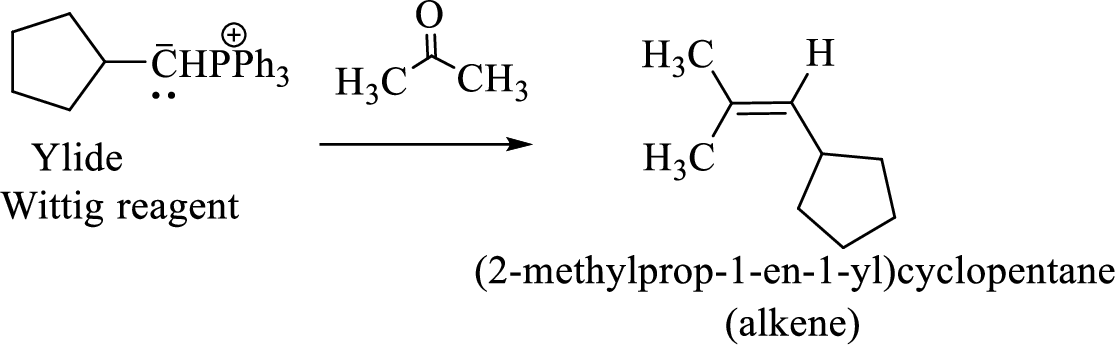
(d)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
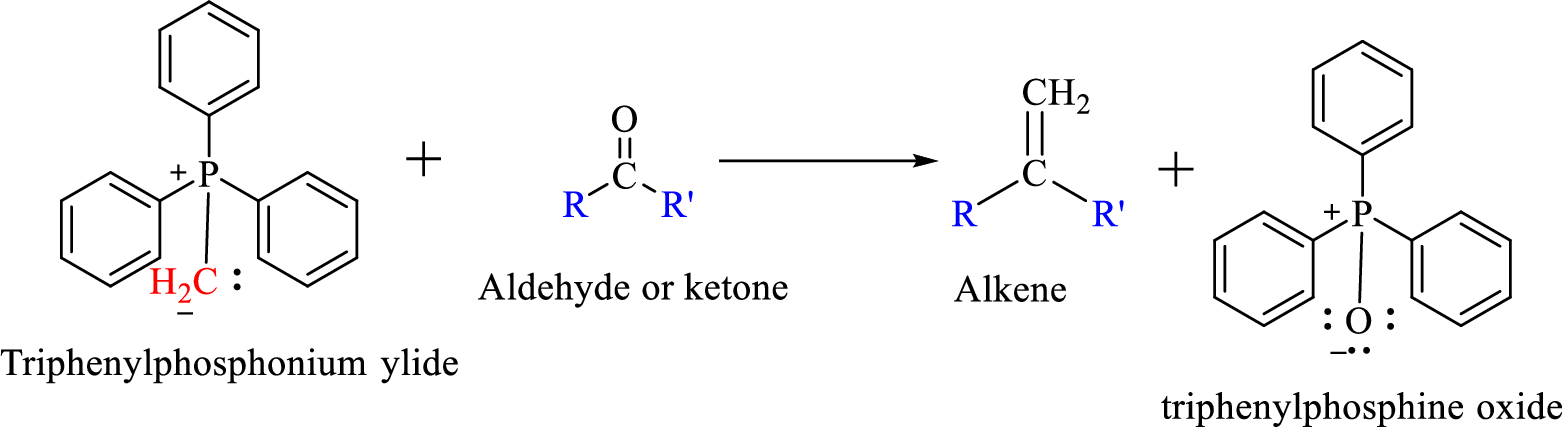
Mechanism:
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.
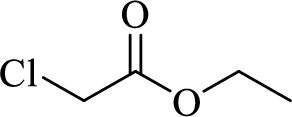
When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
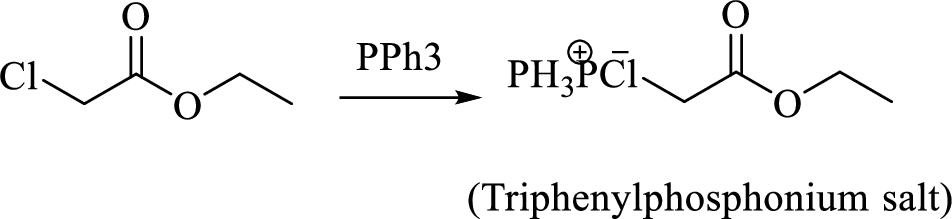
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
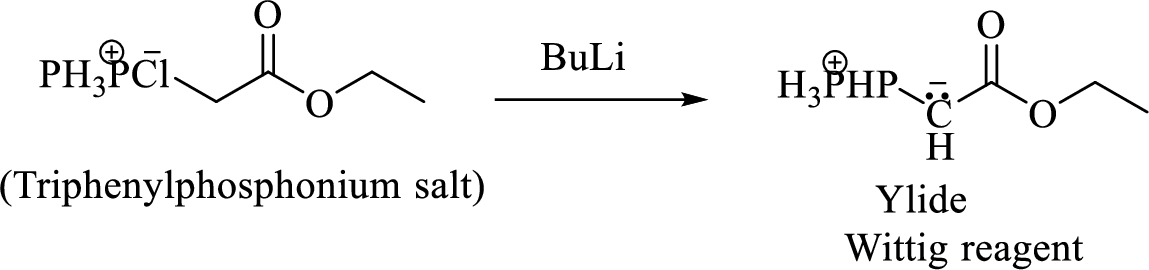
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
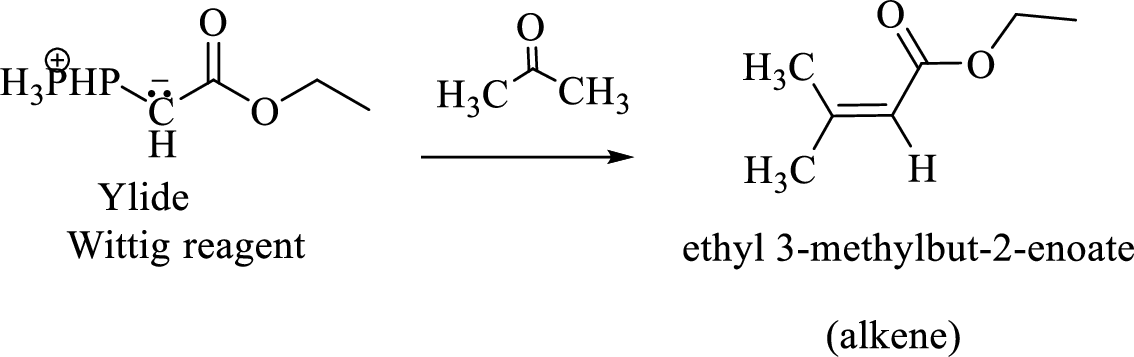
(e)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
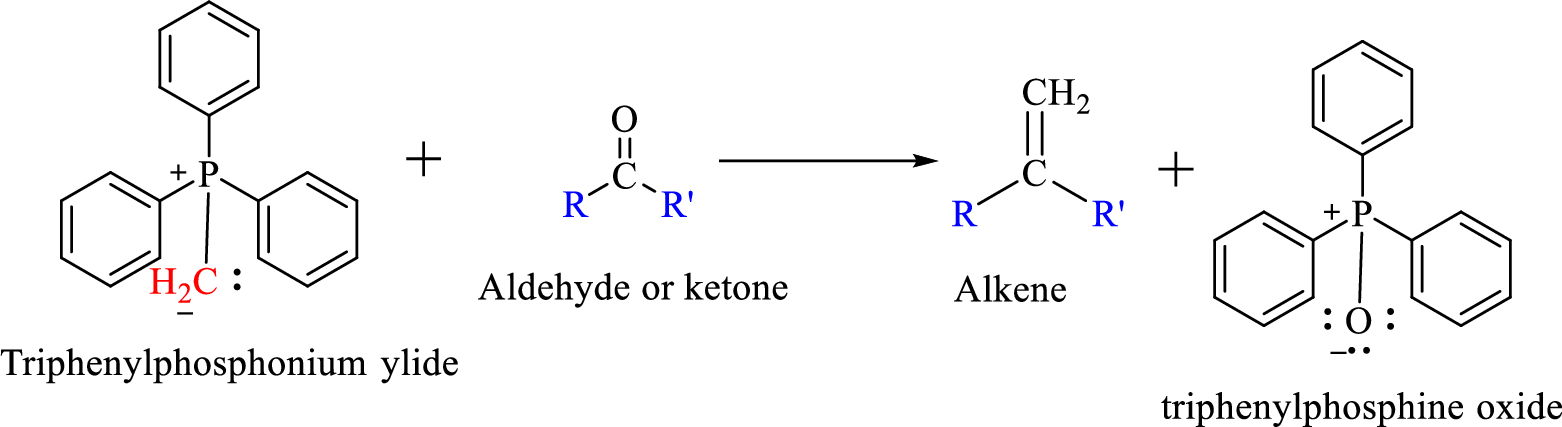
Mechanism:
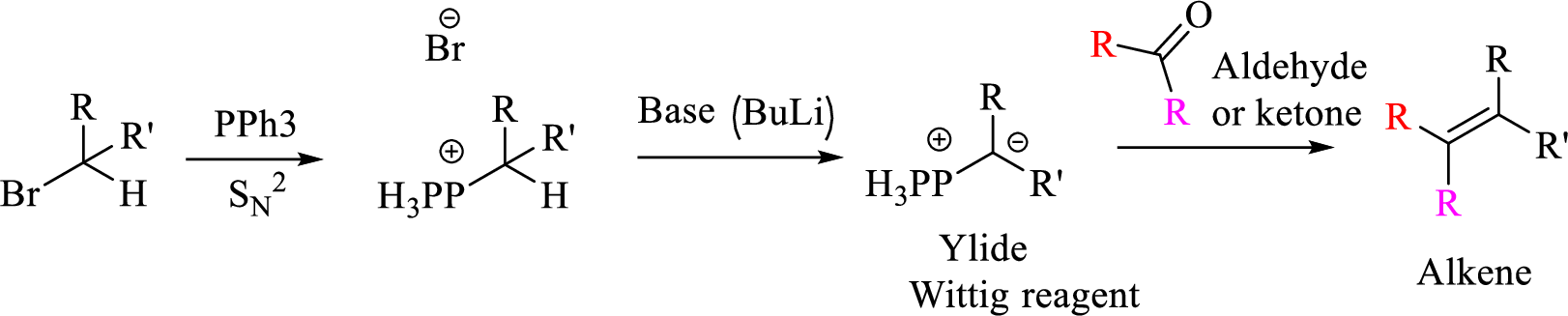
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.

When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
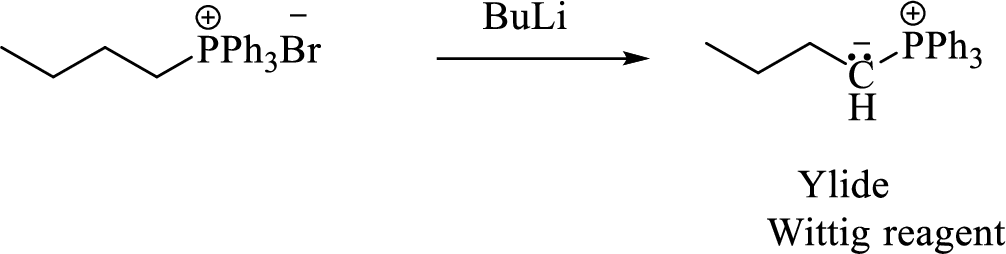
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
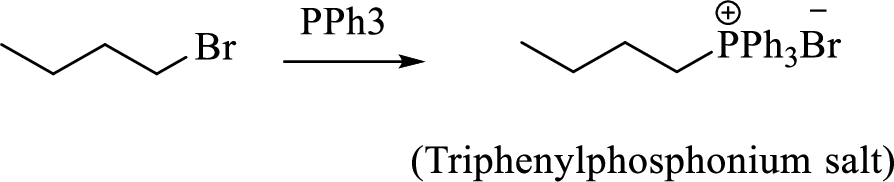
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
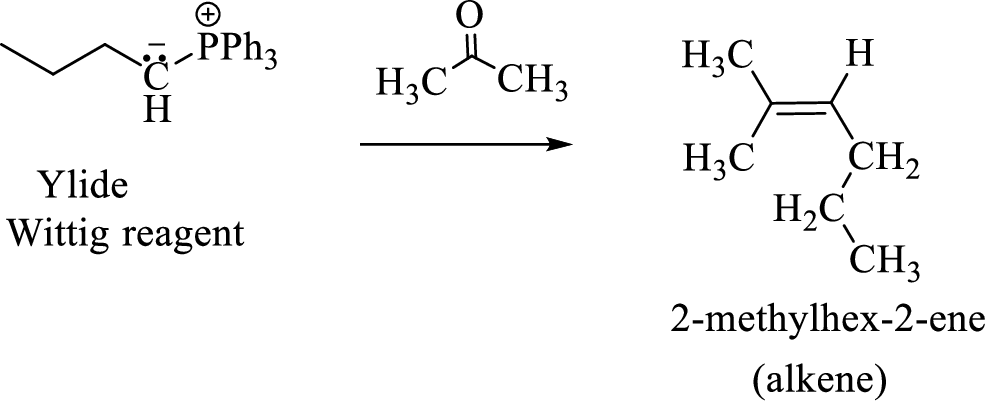
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
(f)
Interpretation:
The structural formulas for the materials formed in the each steps in the Wittig reaction of given reactant has to be drawn.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Wittig reaction is a reaction in which a nucleophilic elimination occurs after nucleophilic addition reaction. In this reaction an aldehyde or ketone reacts with a triphenyl phosphonium ylide (Wittig reagent) to give an alkene and triphenylphosphine oxide.
The reaction can be represented as shown below,
Mechanism:
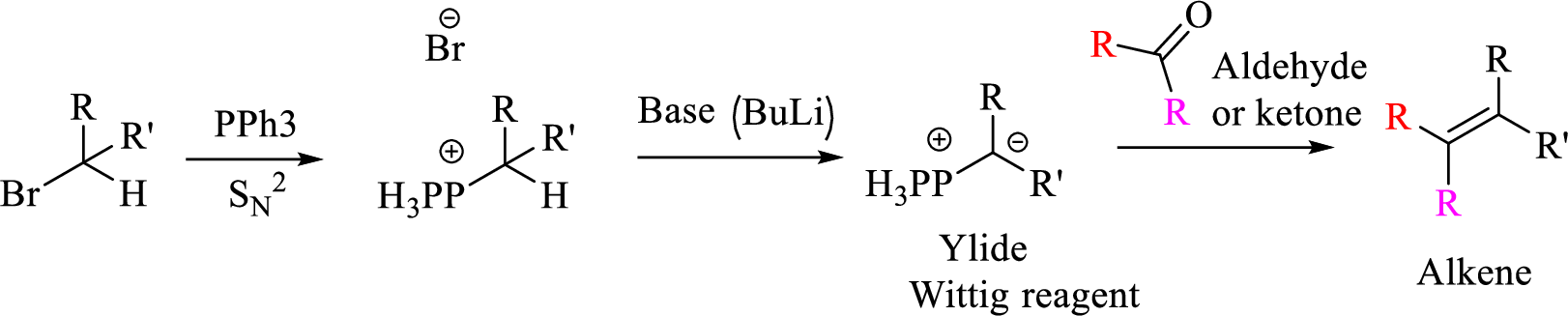
Explanation of Solution
Given halo compound is shown below, 1,2 and 3 in the questions are the important steps in Wittig reaction.

When treating this compound with triphenylphosphine corresponding alkyltriphenylphosphonium salt will form.
Thus, the alkyltriphenylphosphonium salt formed in the reaction (1) can be drawn as follows,
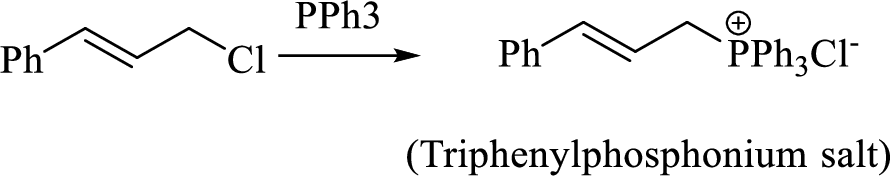
Phosphonium ylide formed by treatment of this phosphonium salt with butyl lithium (2) can be drawn as shown below,
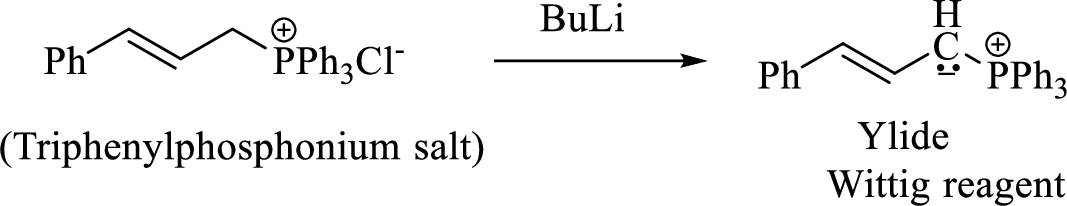
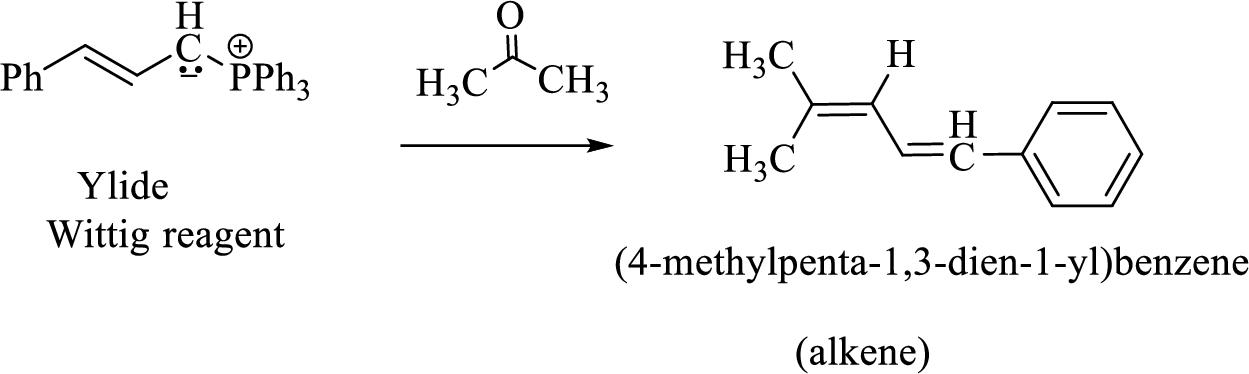
(3) The alkene formed by treatment of this phosphonium ylide with acetone is drawn below,
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry, Loose-leaf Version
Additional Science Textbook Solutions
Physical Universe
Physical Science
Campbell Essential Biology with Physiology (5th Edition)
Microbiology Fundamentals: A Clinical Approach
- (b) Provide the number of peaks in each of the indicated signals ('H NMR) for the compound below. CH3 6 1 H&C. C H₂ H2 3 HA 2 2 4 5 5arrow_forward8. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the ground state from higher energy states. Line A has a wavelength of 10.8 nm. BA Increasing wavelength, \ - a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B? b) Identify the one-electron species that exhibits the spectrum.arrow_forwardShow work with explanation....don't give Ai generated solutionarrow_forward
- achieve.macmillanlearning.com Canvas EA eac h Hulu YouTube G 3 methyl cyclobutanol - Google Search Ranking Phenol Acidity Course -236 - Organic Chemistry - Mac... ← Assessment Completed 10 of 22 Questions 1 + Netflix paramount plus chem hw Galdehyde reaction with grignard reagent... b My Questions | bartleby M Inbox - chenteislegit@gmail.com - Gmail Due: Fri, Jan 31 Resources Solution Penalized ? Hint Submit Answer Use retrosynthetic analysis to suggest two paths to synthesize 2-methyl-3-hexanol using the Grignard reaction. (Click and drag the appropriate image to the correct position in the reactions.) Route 1 Aldehyde 1 or +98 Aldehyde 2 Route 2 Q6 +100 Solved in 1 attempt Q7 +95 Solved in 2 attempts Q8 +98 Unlimited attempts possible + + Grignard 1 OH H3O+ Grignard 2 Answer Bank Q9 +90 MgBr Unlimited attempts possible CH3CH2CH2MgBr Q10 Unlimited attempts Q11 ? ? +100 in 1 attempt 2-methyl-3-hexanol CH3CH2MgBr H H о H Attempt 3arrow_forward2) (4 pt) After the reaction was completed, the student collected the following data. Crude product data is the data collected after the reaction is finished, but before the product is purified. "Pure" product data is the data collected after attempted purification using recrystallization. Student B's data: Crude product data "Pure" product data after recrystallization Crude mass: 0.93 g grey solid Crude mp: 96-106 °C Crude % yield: Pure mass: 0.39 g white solid Pure mp: 111-113 °C Pure % yield: a) Calculate the crude and pure percent yields for the student's reaction. b) Summarize what is indicated by the crude and pure melting points.arrow_forwardDon't used hand raitingarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


