
(a)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
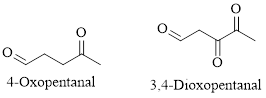
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
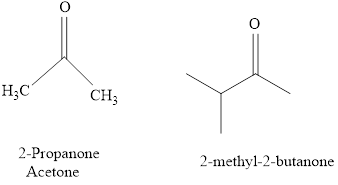
Naming of compounds with two
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(a)
Explanation of Solution
The given compound is as follows.
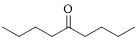
Let’s given numbering to this compound as follows,
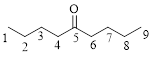
The parent chain contains 9 carbon atoms; in the fifth carbon atom a ketone functional group is attached.
Thus, according to IUPAC this compound can be named as 5-Nonanone.
(b)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
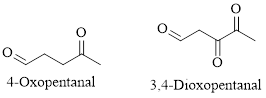
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups,
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(b)
Explanation of Solution
The given compound is as follows.
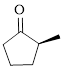
Let’s write give the numbering to this compound.
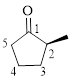
This parent ring has five carbon atoms; a methyl group was attached to the second carbon atom. Therefore, according to the IUPAC rules, the compound can be named as
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
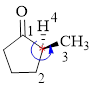
The numbering follows an anti-clock wise direction and so molecule is in as S configuration.
Thus, the compound name can be written as
(c)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
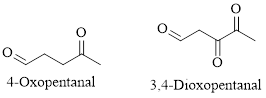
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(c)
Explanation of Solution
The given compound is as follows.
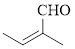
Let’s give the numbering to the given compound.
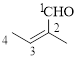
The parent hydrocarbon chain has two functional groups and they are
(d)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
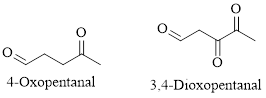
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(d)
Explanation of Solution
The given compound is as follows.
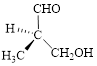
Let’s write give the numbering to this compound.
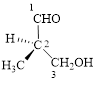
This parent chain has three carbon atoms; a methyl group was attached to the second carbon atom and hydroxyl group was attached to the third carbon atom. Therefore, according to the IUPAC rules, the compound can be named as 3-Hydroxy-2-methyl-propanal.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
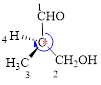
The numbering of substituents on the chiral center follows clock wise direction and so molecule is in R configuration.
Thus, the compound name can be written as (R)-3-Hydroxy-2-methyl-propanal.
(e)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(e)
Explanation of Solution
The given compound is as follows.
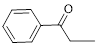
Let’s give the numbering to the given compound.
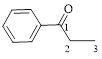
A phenyl ring is attached to the first carbon atom in the three membered parent carbon chains. According to IUPAC the aldehyde group has higher priority. Thus the compound can be named as 1-phenyl-1-propanone.
(f)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
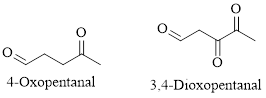
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
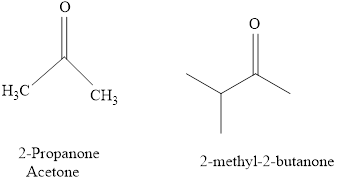
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(f)
Explanation of Solution
The given compound is as follows.
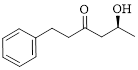
Let’s write give the numbering to this compound.
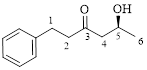
This parent ring has six carbon atoms; a hydroxyl (–OH) and a phenyl ring were attached to the first and fifth carbon atoms in the parent chain respectively. Therefore, according to the IUPAC rules, the compound can be named as 5-Hydroxy-1-phenyl-3-hexanone.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
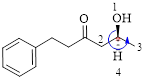
The numbering follows an anti-clock wise direction and so molecule is in as S configuration.
Thus, the compound name can be written as (S)-5-Hydroxy-1-phenyl-3-hexanone.
(g)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(g)
Explanation of Solution
The given compound is as follows.
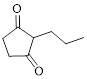
Let’s give the numbering to the given compound.
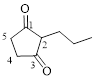
The parent hydrocarbon ring has five carbon atoms. A propyl group was attached to the second carbon atom in the ring and two ketone groups were present in first and third carbon atom respectively. Thus the compound can be named as
(h)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
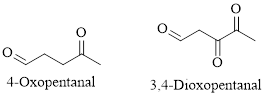
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(h)
Explanation of Solution
The given compound is as follows.
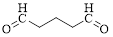
Let’s give the numbering to the given compound.
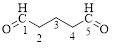
The parent hydrocarbon chain has five carbon atoms with two aldehydes on both ends. Thus according to IUPAC the compound can be named as pentanedial.
(i)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
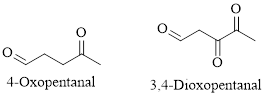
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(i)
Explanation of Solution
The given compound is as follows.
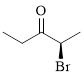
Let’s give the numbering to the given compound.
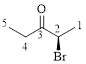
The parent hydrocarbon chain has two functional groups and they are bromine and ketone. According to IUPAC the bromine has higher priority. Thus the compound can be named as 2-bromo-3-pentanone.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
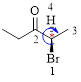
The numbering follows a clock wise direction and so molecule is in R configuration.
Thus, the compound name can be written as (R)-2-bromo-3-pentanone.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry, Loose-leaf Version
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
