
Test Prep Series for AP Chemistry for Chemistry: The Central Science 14th ed AP
14th Edition
ISBN: 9780134661483
Author: Edward L Waterman
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 1E
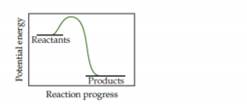
- Based on the following energy profile, predict whether kf > kr or kf < kr.
- Using Equation 15.5, predict whether the equilibrium constant for the process is greater than 1 or less than 1. [Section 15.1]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Consider the following reaction and thermodynamic data.
Compound AH;° (kJ/mol)
(J/mol·K)
3 Ni(s) + N:(g) +3 H:O(g)
3 NiO(s) + 2 NH(g)
NIO (s)
-239.7
38.0
Ni (s)
29.9
a) Calculate AH° for the reaction.
NH3 (g)
-46.0
192.5
b) Calculate AS° for the reaction.
N2 (g)
191.5
c) Determine the standard free energy for the reaction at 25 °C.
H20 (g)
-241.8
188.7
d) Determine the value of the equilibrium constant at 25 °C.
e) Estimate the temperature at which this reaction would be at equilibrium (assuming that enthalpy and
entropy are independent of temperature).
1
Account for the following:
(i) NH3 is a stronger base than PH3.
(ii) Sulphur has a greater tendency for catenation than oxygen.
(iii) Bond dissociation energy of F2 is less than that of Cl?
Chapter 15 Solutions
Test Prep Series for AP Chemistry for Chemistry: The Central Science 14th ed AP
Ch. 15.2 - Prob. 15.1.1PECh. 15.2 - Prob. 15.1.2PECh. 15.2 - Prob. 15.2.1PECh. 15.2 - Prob. 15.2.2PECh. 15.3 - Prob. 15.3.1PECh. 15.3 - Practice Exercise 2 For the reaction H2 (g) + I2...Ch. 15.3 - Prob. 15.4.1PECh. 15.3 - Prob. 15.4.2PECh. 15.4 - Prob. 15.5.1PECh. 15.4 - Prob. 15.5.2PE
Ch. 15.4 - Practice Exercise 1
If 8.0 g of NH4HS(s)...Ch. 15.4 - Prob. 15.6.2PECh. 15.5 - Practice Exercise 1
A mixture of gaseous sulfur...Ch. 15.5 - Prob. 15.7.2PECh. 15.5 - Practice Exercise 1 In Section 15.1, we discussed...Ch. 15.5 - Practice Exercise 2
The gaseous compound BrCl...Ch. 15.6 - Prob. 15.9.1PECh. 15.6 - Practice Exercise 2 At 1000 k, the value of Kp for...Ch. 15.6 - Prob. 15.10.1PECh. 15.6 - Prob. 15.10.2PECh. 15.6 - Practice Exercise 1 For the equilibrium Br2(g) +...Ch. 15.6 - Prob. 15.11.2PECh. 15.7 - Practice Exercise 1 For the reaction 4 NH3(g) + 5...Ch. 15.7 - Prob. 15.12.2PECh. 15 - Prob. 1DECh. 15 - Based on the following energy profile, predict...Ch. 15 - 15.2 The following diagrams represent a...Ch. 15 - Prob. 3ECh. 15 - Prob. 4ECh. 15 - Prob. 5ECh. 15 - 15.6 Ethene (C2H4) reacts with healogens (X2) by...Ch. 15 - When lead(IV) oxide is heated above 300 O C, it...Ch. 15 - Prob. 8ECh. 15 - The reactin A2(g) + B(g) + A(g) + AB(g) has an...Ch. 15 - Prob. 10ECh. 15 - Prob. 11ECh. 15 - The following graph represents the yield of the...Ch. 15 - Suppose that the gas-phase reactions A B and B A...Ch. 15 - Prob. 14ECh. 15 - Prob. 15ECh. 15 - Write the expression for KC for the following...Ch. 15 - When the following reaction come to equilibrium,...Ch. 15 - Prob. 18ECh. 15 - Prob. 19ECh. 15 - Prob. 20ECh. 15 - If Kc = 0.042 for PC13(g) + C12 (g) PC15 (g) at...Ch. 15 - Prob. 22ECh. 15 - 15.23 The equilibrium constant for the...Ch. 15 - Prob. 24ECh. 15 - Prob. 25ECh. 15 - Prob. 26ECh. 15 - The following equilibria were attained at 823 K:...Ch. 15 - Consider the equilibrium N2(g) + O2(g) + Br2(g) 2...Ch. 15 - Mercury(I) oxide decomposes into elemental mercury...Ch. 15 - Prob. 30ECh. 15 - Prob. 31ECh. 15 - Prob. 32ECh. 15 - Prob. 33ECh. 15 - Phosphorus trichloride gas and chlorine gas react...Ch. 15 - A mixture of 0.10 mol of NO, 0.050 mol of H2, and...Ch. 15 - Prob. 36ECh. 15 - A mixture of 0.2000 mol of CO2, 0.1000 mol of H2,...Ch. 15 - 15.38 A flask is charged with 1.500 atm of N2O4(g)...Ch. 15 - Prob. 39ECh. 15 - Prob. 40ECh. 15 - a. If QC KC, in which direction will a reaction...Ch. 15 - Prob. 42ECh. 15 - At 100 OC , the equilibrium constant for the...Ch. 15 - 15.44 As shown in Table 15.2, KP for the...Ch. 15 - At 100 C, K = 0.078 for the reaction SO2Cl2 (g) ...Ch. 15 - Prob. 46ECh. 15 - Prob. 47ECh. 15 - Prob. 48ECh. 15 - At 800 k, the equilibrium constant for I2 (g) ...Ch. 15 - Prob. 50ECh. 15 - At 2000 OC, the equilibrium constant for the...Ch. 15 - For the equilibrium Br2 (g) + Cl2 (g) 2BrCl(g) At...Ch. 15 - At 373 k, Kp = 0.416 for the equilibrium 2NOBr (g)...Ch. 15 - At 218 oC, KC= 1.2 X 10-4 for the equilibrium NH4...Ch. 15 - Prob. 55ECh. 15 - At 80 oC, K =1.87 X 10-3 for the reaction PH3 BCl3...Ch. 15 - Prob. 57ECh. 15 - Prob. 58ECh. 15 - Prob. 59ECh. 15 - Prob. 60ECh. 15 - Consider the following equilibrium for which H<0...Ch. 15 - Prob. 62ECh. 15 - 15.63 How do the following changes affect the...Ch. 15 - Prob. 64ECh. 15 - Consider the following equilibrium between oxides...Ch. 15 - Prob. 66ECh. 15 - Ozone, O3, decomposes to molecular oxygen in the...Ch. 15 - Prob. 68ECh. 15 - Prob. 69ECh. 15 - 15.70 True or false: When the temperature of an...Ch. 15 - Prob. 71AECh. 15 - Prob. 72AECh. 15 - 15.73 A mixture of CH4 and H2O is passed over a...Ch. 15 - Prob. 74AECh. 15 - Prob. 75AECh. 15 - Prob. 76AECh. 15 - Prob. 77AECh. 15 - Prob. 78AECh. 15 - Prob. 79AECh. 15 - For the equilibrium PH3BCI3 (s) PH3 (g) + BCI3...Ch. 15 - Prob. 81AECh. 15 - Prob. 82AECh. 15 - Prob. 83AECh. 15 - At 900 o C, Kc = 0.0108 for the reaction CaCO3(g) ...Ch. 15 - Prob. 85AECh. 15 - The equilibrium constant Kc for C(s) +CO2 2CO(g)...Ch. 15 - Prob. 87AECh. 15 - Le Chatelier noted that many industrial processes...Ch. 15 - Prob. 89AECh. 15 - Prob. 90AECh. 15 - [15.91] An equilibrium mixture of H2, I2, and HI...Ch. 15 - Consider the hypothetical reaction A(g) + 2B(g) 2...Ch. 15 - Prob. 93AECh. 15 - Prob. 94AECh. 15 - Prob. 95IECh. 15 - The following equilibria were measured at 823 K:...Ch. 15 - Prob. 97IECh. 15 - Prob. 98IECh. 15 - At 800 K, the equilibrium constant for the...Ch. 15 - Prob. 100IECh. 15 - Prob. 101IECh. 15 - Prob. 102IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Gibbs Free Energy for Nonspontaneous Reactions and Reactions at Equilibrium Consider the given Ellingham diagram. Rank the oxides of Mg, Zn, and Al in terms of increasing stability at 1000 ºC (1 being the least stable, 3 being the most stable).arrow_forwardConsider the equilibrium, CO2(g) + H2(g) < ---- > CO(g) + H2O(g). Calculate the equilibrium concentration of CO, given that the equilibrium constant, Kc, is 0.0049 and the equilibrium concentrations of H2O(g) is 0.46 M, that of H2(g) is 4.54 M, and that of CO2(g) is 9.54 M. Group of answer choices 0.46 M 10. M 0.098 M 2.2 Marrow_forwardDescribe various methods for synthesis of cobalt(II) sulphate (CoSO4.7H2O) in detail.arrow_forward
- Given the following equilibrium: 2 SO2(g) + O2(g) ↔ 2 SO3(g) + heat State 3 conditions that will favour a high concentration of SO3 at equilibrium.arrow_forwardWrite the expression for the equilibrium constant Kc for the following equation. FeO(s) + CO(g) ↔ Fe(s) + CO2(g)arrow_forwardThe interhalogen compound BrF3 is a volatile, straw-colored liquid. The compound exhibits appreciable electrical conductivitybecause of autoionization (“solv” refers to BrF3 as the solvent):2 BrF3(l) ⇌ BrF2+(solv) + BrF4- (solv)(a) What are the molecular structures of the BrF2+ and BrF4- ions?(b) The electrical conductivity of BrF3 decreases with increasing temperature. Is the autoionization process exothermic or endothermic?(c) One chemical characteristic of BrF3 is that it acts as a Lewis acid toward fluoride ions. What do we expect will happen whenKBr is dissolved in BrF3?arrow_forward
- 1. Write the reaction quotient, Qc. for each of the following reactions: (a) The first step in nitric acid production, NH3(g) + O2(g) NO(g) + H₂O(g) (b) The disproportionation of nitrogen monoxide, NO(g) N₂O(g) + NO₂(g)arrow_forward1. Write the equilibrium constant for 3NO(g) ↔ N2O(g) + NO2(g)arrow_forward1. Suggest why it is possible to synthesize many boron-nitride analogs of aromatic carbon compounds, such as white graphite (the BN analog of graphite): Boron nitride Graphite 2. Provide the balanced chemical reaction for the Haber-Bosch process: Do you expect this is exothermic or endothermic and why?arrow_forward
- (a) Suppose that CaO is added as an impurity to Li2O. If the Ca2+ substitutes for Li+ , what kind of vacancies would you expect to form? How many of these vacancies are created for every Ca2+ added? (b) Suppose that CaO is added as an impurity to CaCl2 . If the O2– substitutes for Cl– , what kind of vacancies would you expect to form? How many of these vacancies are created for every O2– added?arrow_forwardWhat is the degree of ionization (i) for Na₃PO₄(aq)?arrow_forwardConsider metal sulfites (MSO3) – these decompose upon heating to form the corresponding metal oxide (MO) and SO2 (note: M is a group II metal). Based on this information, please explain how the stability of the metal sulfites trends within the group and justify your answer. Which would you expect to be more stable: Na2SO3 or MgSO3?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY