
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.71P
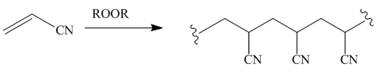
Draw a stepwise mechanism for the following
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
2'
P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by
the following mechanism:
NO + NO → N₂O₂
k₁
N2O2 NO NO
K
=
N2O2 + O2 → NO2 + NO₂
Ко
Verify that application of the steady-state approximation to the intermediate
N2O2 results in the rate law
d[NO₂] _ 2kk₁[NO][O₂]
=
dt
k+k₁₂[O₂]
PLEASE ANSWER BOTH i) and ii) !!!!
Chapter 15 Solutions
Organic Chemistry
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Prob. 15.3PCh. 15 - Prob. 15.4PCh. 15 - Prob. 15.5PCh. 15 - Problem 15.6 Using mechanism 15.1 as guide, write...Ch. 15 - Prob. 15.7PCh. 15 - Problem 15.8 Which bond in the each compound is...Ch. 15 - Prob. 15.9PCh. 15 - Prob. 15.10P
Ch. 15 - Prob. 15.11PCh. 15 - Prob. 15.12PCh. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - Prob. 15.15PCh. 15 - Prob. 15.16PCh. 15 - Draw the products of each reaction.
a. b. c.
Ch. 15 - Draw all constitutional isomers formed when each...Ch. 15 - Draw the structure of the four allylic halides...Ch. 15 - Problem 15.20 Which compounds can be prepared in...Ch. 15 - Which CH bond is most readily cleaved in linolenic...Ch. 15 - Prob. 15.22PCh. 15 - Prob. 15.23PCh. 15 - Problem 15.24 When adds to under radical...Ch. 15 - Prob. 15.25PCh. 15 - Prob. 15.26PCh. 15 - Problem 15.27 Draw the steps of the mechanism that...Ch. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - 15.35 What is the major monobromination product...Ch. 15 - Prob. 15.36PCh. 15 - 15.37 What alkane is needed to make each alkyl...Ch. 15 - 15.38 Which alkyl halides can be prepared in good...Ch. 15 - Prob. 15.39PCh. 15 - 15.40 Explain why radical bromination of p-xylene...Ch. 15 - a. What product(s) (excluding stereoisomers) are...Ch. 15 - Prob. 15.42PCh. 15 - 15.43 Draw the products formed when each alkene is...Ch. 15 - 15.44 Draw all constitutional isomers formed when...Ch. 15 - 15.45 Draw the organic products formed in each...Ch. 15 - Prob. 15.46PCh. 15 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 15 - 15.48 Draw the products formed in each reaction...Ch. 15 - Prob. 15.49PCh. 15 - 15.50 Draw all the monochlorination products that...Ch. 15 - Prob. 15.51PCh. 15 - 15.52 (a) Draw the products (including...Ch. 15 - 15.53 Consider the following bromination: .
a....Ch. 15 - 15.54 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 15.55PCh. 15 - Prob. 15.56PCh. 15 - 15.57 Devise a synthesis of each compound from...Ch. 15 - Prob. 15.58PCh. 15 - Prob. 15.59PCh. 15 - 15.60 Devise a synthesis of each compound using ...Ch. 15 - Prob. 15.61PCh. 15 - Prob. 15.62PCh. 15 - 15.63 As described in Section 9.16, the...Ch. 15 - 15.64 Ethers are oxidized with to form...Ch. 15 - Prob. 15.65PCh. 15 - Prob. 15.66PCh. 15 - 15.67 In cells, vitamin C exists largely as its...Ch. 15 - What monomer is needed to form each...Ch. 15 - Prob. 15.69PCh. 15 - Prob. 15.70PCh. 15 - 15.71 Draw a stepwise mechanism for the following...Ch. 15 - 15.72 As we will learn in Chapter 30, styrene...Ch. 15 - Prob. 15.73PCh. 15 - 15.74 A and B, isomers of molecular formula , are...Ch. 15 - Prob. 15.75PCh. 15 - 15.76 Draw a stepwise mechanism for the...Ch. 15 - Prob. 15.77PCh. 15 - Prob. 15.78PCh. 15 - Prob. 15.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- E17E.2(a) The following mechanism has been proposed for the decomposition of ozone in the atmosphere: 03 → 0₂+0 k₁ O₁₂+0 → 03 K →> 2 k₁ Show that if the third step is rate limiting, then the rate law for the decomposition of O3 is second-order in O3 and of order −1 in O̟.arrow_forward10.arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
- 2arrow_forwardWhich of the following starting materials and reagents would be best to produce a racemic mixture of 3-methyl-3-hexanol? heptanone and 1. CH3MgBr 2. H3O+ hexanal and 1. CH3MgBr, 2. H3O+ 3-hexanone and 1. CH3MgBr, 2. H3O+ butanal and 1. CH3CH2MgBr, 2. H3O+arrow_forwardCan someone draw a reaction mechanism of this reaction please I was told that the boc l alanine is deprotonated first and acts as the nucleophile attacking the EDCL and can you please show all the intermediates and side products and the water at the endarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY