
Concept explainers
(a)
Interpretation:
Whether the given compound is optically active or not needs to be determined.
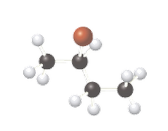
Concept Introduction:
The ball-stick model is a method of representation of molecules to demonstrate the three-dimensional position of atoms and the bonds. The atoms are represented by different colored spheres, while bonds are represented by rods connecting the spheres. Chiral or optically active molecules can rotate plane-polarized light either clockwise or counterclockwise. If plane-polarized light is rotated clockwise then it is known as dextrorotatory and for counterclockwise rotation, it is known as levorotatory.
(b)
Interpretation:
Whether the given compound is optically active or not needs to be determined.
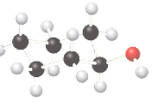
Concept Introduction:
The ball-stick model is a method of representation of molecules to demonstrate the three-dimensional position of atoms and the bonds. The atoms are represented by different colored spheres, while bonds are represented by rods connecting the spheres. Chiral or optically active molecules can rotate plane-polarized light either clockwise or counterclockwise. If plane-polarized light is rotated clockwise then it is known as dextrorotatory and for counterclockwise rotation, it is known as levorotatory.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. ? NH2 MgBr Will the first product that forms in this reaction create a new CC bond? ○ Yes ○ No MgBr ? Will the first product that forms in this reaction create a new CC bond? O Yes O No Click and drag to start drawing a structure. :☐ G x c olo Ar HEarrow_forwardPredicting As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: H₂N O H 1. ? 2. H3O+ If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. 0 If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. فا Explanation Check Click and drag to start drawing a structure.arrow_forwardHighlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers. OH OH OH OH OH OHarrow_forward
- Using wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
- Given a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

