
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.43P
15-43 Triamcinolone acetonide, the active ingredient in Azmacort Inhalation Aerosol, is a steroid used to treat bronchial asthma.
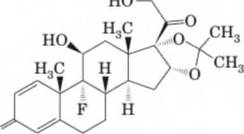
Triamcinolone acetonide
- Label the eight stereocenters in this molecule.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
9. OA. Rank the expected boiling points of the compounds shown below from highest to lowest. Place your answer
appropriately in the box. Only the answer in the box will be graded. (3) points)
OH
OH
بر بد بدید
2
3
There is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS).
Ca, ppm
V, ppm
SCa, arb. units
SV, arb. units
20.0
10.0
14375.11
14261.02
40.0
10.0
36182.15
17997.10
60.0
10.0
39275.74
12988.01
80.0
10.0
57530.75
14268.54
100.0…
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
Ch. 15.1 - Prob. 15.1PCh. 15.2 - Problem 15-2 Assign priorities to the groups in...Ch. 15.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 15.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 15.3 - Prob. 15.5PCh. 15.3 - Prob. 15.6PCh. 15 - 15-7 Answer true or false. The cis and trans...Ch. 15 - 15-8 What does the term “chiral” mean? Give an...Ch. 15 - 15-9 What does the term “achiral” mean? Give an...Ch. 15 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 15 - 15-11 In what way are constitutional isomers...Ch. 15 - 15-12 Which of the following objects are chiral...Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - 15-15 Explain why the carbon of a carbonyl group...Ch. 15 - 15-16 Which of the following compounds contain...Ch. 15 - 15-17 Which of the following compounds contain...Ch. 15 - Prob. 15.18PCh. 15 - 15-19 Draw the mirror image for each molecule: OH...Ch. 15 - Prob. 15.20PCh. 15 - 15-21 Answer true or false. For a molecule with...Ch. 15 - Prob. 15.22PCh. 15 - Prob. 15.23PCh. 15 - Prob. 15.24PCh. 15 - Prob. 15.25PCh. 15 - 15-26 For centuries, Chinese herbal medicine has...Ch. 15 - Prob. 15.27PCh. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - 15-30 (Chemical Connections 15A) What does it mean...Ch. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - 15-35 Following are structural formulas for three...Ch. 15 - Prob. 15.36PCh. 15 - 15-37 Consider a cyclohexane ring substituted with...Ch. 15 - Prob. 15.38PCh. 15 - Prob. 15.39PCh. 15 - Prob. 15.40PCh. 15 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 15 - Prob. 15.42PCh. 15 - 15-43 Triamcinolone acetonide, the active...Ch. 15 - 15-44 Consider the structure of the...Ch. 15 - Prob. 15.45PCh. 15 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 15 - Prob. 15.47P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forwardWhat units (if any) does K have? Does K depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)? in calculating the response factorarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forwardOA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forward
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY