
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.26P
15-26 For centuries, Chinese herbal medicine has
used extracts oi Ephedra sinica to treat asthma. The asthma-relieving component of this plant is ephedrine, a very potent dilator of the air passages of the lungs. The naturally occurring stereoisomer is levorotatory and has the following structure.
HO ¥ Nhch3
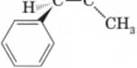
Ephedrine |n|„ = —41°
- Mark each stereocenter in epinephrine with an asterisk.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I just need help with A,F,G,H
QUESTION 1
Write the IUPAC names for the following compounds.
(a)
(b)
2
H₂C
CH
(c)
Br
(d)
HO
(e)
COOH
need help finding the product of these reactions
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
Ch. 15.1 - Prob. 15.1PCh. 15.2 - Problem 15-2 Assign priorities to the groups in...Ch. 15.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 15.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 15.3 - Prob. 15.5PCh. 15.3 - Prob. 15.6PCh. 15 - 15-7 Answer true or false. The cis and trans...Ch. 15 - 15-8 What does the term “chiral” mean? Give an...Ch. 15 - 15-9 What does the term “achiral” mean? Give an...Ch. 15 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 15 - 15-11 In what way are constitutional isomers...Ch. 15 - 15-12 Which of the following objects are chiral...Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - 15-15 Explain why the carbon of a carbonyl group...Ch. 15 - 15-16 Which of the following compounds contain...Ch. 15 - 15-17 Which of the following compounds contain...Ch. 15 - Prob. 15.18PCh. 15 - 15-19 Draw the mirror image for each molecule: OH...Ch. 15 - Prob. 15.20PCh. 15 - 15-21 Answer true or false. For a molecule with...Ch. 15 - Prob. 15.22PCh. 15 - Prob. 15.23PCh. 15 - Prob. 15.24PCh. 15 - Prob. 15.25PCh. 15 - 15-26 For centuries, Chinese herbal medicine has...Ch. 15 - Prob. 15.27PCh. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - 15-30 (Chemical Connections 15A) What does it mean...Ch. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - 15-35 Following are structural formulas for three...Ch. 15 - Prob. 15.36PCh. 15 - 15-37 Consider a cyclohexane ring substituted with...Ch. 15 - Prob. 15.38PCh. 15 - Prob. 15.39PCh. 15 - Prob. 15.40PCh. 15 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 15 - Prob. 15.42PCh. 15 - 15-43 Triamcinolone acetonide, the active...Ch. 15 - 15-44 Consider the structure of the...Ch. 15 - Prob. 15.45PCh. 15 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 15 - Prob. 15.47P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardUsing the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forward
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY