
(a)
Interpretation:
To draw the mirror image of the given molecule.
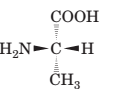
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. Stereoisomers which don't have any mirror image relationship are called diastereomers.
(b)
Interpretation:
To draw the mirror image of the given molecule.
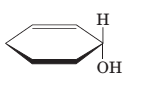
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. Stereoisomers which don't have any mirror image relationship are called Diastereomers.
(c)
Interpretation:
To draw the mirror image of the given molecule.
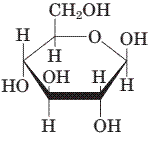
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. Stereoisomers which don't have any mirror image relationship are called Diastereomers.
(d)
Interpretation:
To draw the mirror image of the given molecule.
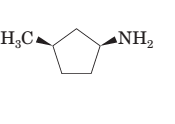
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. Stereoisomers which don't have any mirror image relationship are called Diastereomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
- 5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forwardThe mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward
- 32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forwardDon't used hand raitingarrow_forward
- Can you please explain why the answer is structures 2 and 3? Please include a detailed explanation and show how the synthesis can be done with those two structures.arrow_forwardCan you please explain why the correct answer to this question is option 2? I am having trouble understanding how and why. Please provide a detailed explanation and a drawing of how the diene and dienophile would create the product in the question.arrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Base your explanation off of the rules for aromaticity and well as the principles of the Huckel rule of aromaticity. Please give a detailed explanation of what Hucekl's rule is.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





