
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
9th Edition
ISBN: 9781305968707
Author: Spencer L. Seager
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.37E
Using the alcohol
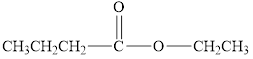
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
Problem 164 of
N
Select to Add Arrows
CHI
CH
1
1
1
P
using these can you help me , I guess convert them to lewis dit structures or full drawn out skeletal and I guess is that what would help me depict the bond angle.
Show reaction mechanism with explanation.don't give Ai generated solution
Chapter 15 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
Ch. 15 - Prob. 15.1ECh. 15 - Prob. 15.2ECh. 15 - Prob. 15.3ECh. 15 - What carboxylic acid is present in sour milk and...Ch. 15 - Write the correct IUPAC name for each of the...Ch. 15 - Write the correct IUPAC name for each of the...Ch. 15 - Prob. 15.7ECh. 15 - Prob. 15.8ECh. 15 - Prob. 15.9ECh. 15 - Prob. 15.10E
Ch. 15 - Prob. 15.11ECh. 15 - Prob. 15.12ECh. 15 - Prob. 15.13ECh. 15 - Caproic acid, a six-carbon acid, has a solubility...Ch. 15 - Why are acetic acid, sodium acetate, and sodium...Ch. 15 - List the following compounds in order of...Ch. 15 - Prob. 15.17ECh. 15 - Prob. 15.18ECh. 15 - Prob. 15.19ECh. 15 - Prob. 15.20ECh. 15 - Write an equation to illustrate the equilibrium...Ch. 15 - Prob. 15.22ECh. 15 - Complete each of the following reactions: a. b.Ch. 15 - Prob. 15.24ECh. 15 - Write a balanced reaction for the reaction of...Ch. 15 - Prob. 15.26ECh. 15 - Give the IUPAC name for each of the following: a....Ch. 15 - Prob. 15.28ECh. 15 - Prob. 15.29ECh. 15 - Prob. 15.30ECh. 15 - Prob. 15.31ECh. 15 - Give the name of a carboxylic acid or carboxylate...Ch. 15 - Prob. 15.33ECh. 15 - Prob. 15.34ECh. 15 - Complete the following reactions: a. b. c.Ch. 15 - Prob. 15.36ECh. 15 - Using the alcohol CH3CH2OH, show three different...Ch. 15 - Prob. 15.38ECh. 15 - Prob. 15.39ECh. 15 - Prob. 15.40ECh. 15 - Prob. 15.41ECh. 15 - Prob. 15.42ECh. 15 - Prob. 15.43ECh. 15 - Prob. 15.44ECh. 15 - Give the IUPAC name for each of the following: a....Ch. 15 - Prob. 15.46ECh. 15 - Prob. 15.47ECh. 15 - Prob. 15.48ECh. 15 - Prob. 15.49ECh. 15 - Prob. 15.50ECh. 15 - Prob. 15.51ECh. 15 - Prob. 15.52ECh. 15 - Complete the following reactions: a. b.Ch. 15 - Prob. 15.54ECh. 15 - Prob. 15.55ECh. 15 - Dihydroxyacetone reacts with phosphoric acid to...Ch. 15 - Prob. 15.57ECh. 15 - Prob. 15.58ECh. 15 - Prob. 15.59ECh. 15 - Prob. 15.60ECh. 15 - How many mL of a 0.100M NaOH solution would be...Ch. 15 - Prob. 15.62ECh. 15 - Prob. 15.63ECh. 15 - Prob. 15.64ECh. 15 - Prob. 15.65ECh. 15 - Prob. 15.66ECh. 15 - Prob. 15.67ECh. 15 - Why is it safe for us to consume foods like...Ch. 15 - Prob. 15.69ECh. 15 - Prob. 15.70ECh. 15 - Prob. 15.71ECh. 15 - Prob. 15.72ECh. 15 - Identify the functional group designated by each...Ch. 15 - Prob. 15.74ECh. 15 - Fats belong to the class of organic compounds...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the questions and provide detailed explanations.arrow_forwardShow reaction mechanism. Don't give Ai generated solutionarrow_forwardPlease answer the questions and provide detailed explanation. Please also include the Hydrogens that are on the molecule to show how many signals there are.arrow_forward
- Capp aktiv.com Part of Speech Table for Assi x Aktiv Learning App K Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 232 of 10 10: Mg Select to Add Arrows Br O H :0 CI:O H Mg THE + dy Undo Reset Done Brarrow_forwardPlease answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forwardNeed help with witharrow_forward
- Please answer the questions and provide detailed explanations.arrow_forwardsolve pleasearrow_forwardPlease answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forward
- Please do not use AI. AI cannot "see" the molecules properly, and it therefore gives the wrong answer while giving incorrect descriptions of the visual images we're looking at. All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forwardPlease answer the question and provide detailed explanations.arrow_forwardAll of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY