
Biochemistry
8th Edition
ISBN: 9781285429106
Author: Campbell, Mary K., FARRELL, Shawn O.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15, Problem 13RE
Interpretation Introduction
Interpretation:
The influence on the concentrations of reactants and products on reactions is to be explained in question  .
.
Concept Introduction:
Gibbs free energy is the standard free energy which complements the production of  mole of that substance from its component elements, at their standard states.
mole of that substance from its component elements, at their standard states.
The symbol of standard Gibbs free energy is 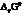 .
.
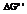 is the measurement of Gibbs free energy which is used to do work done at
is the measurement of Gibbs free energy which is used to do work done at  and
and 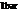 .
.
Gibbs free energy equation is 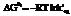
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sodium borohydride (NaBH4) is a potent inhibitor of aldolase. It is known to ONLY inhibit theenzyme when it is complexed with substrate. Treatment of the enzyme alone has no effect.What is the mechanism for this inhibition?
A non-hydrolysable ATP (AMPPNP - below) is ingested by a graduate student on a dare. Whateffects would you anticipate on his metabolism?
Show the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.
Please sketch the structure and use arrows showing electron flow!
Chapter 15 Solutions
Biochemistry
Ch. 15 - RECALL Is there a connection between the...Ch. 15 - REFLECT AND APPLY What do the following indicators...Ch. 15 - REFLECT AND APPLY Consider the reaction...Ch. 15 - RECALL What conditions are necessary for the...Ch. 15 - REFLECT AND APPLY Why is it important that energy...Ch. 15 - RECALL Why is it necessary to define a modified...Ch. 15 - RECALL Which of the following statements is (are)...Ch. 15 - RECALL How can you tell if the standard Gibbs free...Ch. 15 - RECALL Can the thermodynamic property G be used to...Ch. 15 - MATHEMATICAL Calculate G for the following values...
Ch. 15 - Prob. 11RECh. 15 - MATHEMATICAL Consider the reaction AB+C, where...Ch. 15 - Prob. 13RECh. 15 - MATHEMATICAL The G for the reaction Citrate ...Ch. 15 - MATHEMATICAL If a reaction can be written AB, and...Ch. 15 - Prob. 16RECh. 15 - Prob. 17RECh. 15 - Prob. 18RECh. 15 - RECALL Organize the following words into two...Ch. 15 - Prob. 20RECh. 15 - REFLECT AND APPLY Would you expect the production...Ch. 15 - Prob. 22RECh. 15 - REFLECT AND APPLY Adult humans synthesize large...Ch. 15 - RECALL Identify the molecules oxidized and reduced...Ch. 15 - RECALL For each of the reactions in Question 24,...Ch. 15 - Prob. 26RECh. 15 - RECALL What is the structural difference between...Ch. 15 - RECALL How does the difference between NADH and...Ch. 15 - RECALL Which coenzyme is a reactant in the...Ch. 15 - Prob. 30RECh. 15 - Prob. 31RECh. 15 - Prob. 32RECh. 15 - REFLECT AND APPLY The following half reactions...Ch. 15 - Prob. 34RECh. 15 - REFLECT AND APPLY There is a reaction in...Ch. 15 - REFLECT AND APPLY There is a reaction in which...Ch. 15 - Prob. 37RECh. 15 - Prob. 38RECh. 15 - Prob. 39RECh. 15 - Prob. 40RECh. 15 - MATHEMATICAL Using the data in Table 15.1,...Ch. 15 - Prob. 42RECh. 15 - Prob. 43RECh. 15 - MATHEMATICAL The standard free-energy change for...Ch. 15 - Prob. 45RECh. 15 - Prob. 46RECh. 15 - Prob. 47RECh. 15 - REFLECT AND APPLY Would you expect an increase or...Ch. 15 - REFLECT AND APPLY Explain and show why...Ch. 15 - Prob. 50RECh. 15 - Prob. 51RECh. 15 - Prob. 52RECh. 15 - Prob. 53RECh. 15 - REFLECT AND APPLY Why are thioesters considered...Ch. 15 - Prob. 55RECh. 15 - REFLECT AND APPLY This is a conjectural question:...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- I am a Biochemistry student and I am confused on how to analyze FRAP Analysis using Excel Spread Sheets. The following spread sheet has my 0 minute data listed at top and the 4 minute data listed on the bottom. Sheet: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EXjrCizWiXRPmpittqZA12IB8EkB5eE8iaRqj_iun-IAtg?rtime=Wo9zPHFY3Ug The formula for FRAP Analysis is: FRAP value = A (4 min sample) - A (0 min sample) over A (4 min 30 uM ascorbic acid) - A (0 min 30 uM ascorbic acid) multiplied by 30 uM and the dilution factor of 1/10arrow_forwardHO Fill in the missing boxes. ON 800 NO NO Glucose ATP NADH Hexokinase 1,3-Bisphosphoglycerate Mg2+ ADP NAD+, Pi Phosphoglucose Isomerase Glucose-6-Phosphate ON 沁 Glyceraldehyde-3-Phosphate HO حلمة ADP ADP Phospho Mg2+ glycerate Dihydroxyacetone Phosphate ATP kinase ATP Phosphoglycerate 3-phosphoglycerate Mutase H₁₂O Fructose-6-Phosphate ATP Mg2+ ADP Fructose-1,6-Bisphosphate 2-phosphoglycerate H₂O Phosphoenolpyruvate ADP Mg2+ ATP Pyruvatearrow_forwardIn a diffraction experiment of a native crystal, intensity of reflection (-1 0 6) is equivalent to the intensity of reflection (1 0 -6). true or false?arrow_forward
- in an x-ray diffraction experiment, moving the detector farther away from the crystal will allow collection of reflection of reflections with high Miller indices. true or false?arrow_forwardShow the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.arrow_forwardLabel the following polysaccharide derivatives as reducing or nonreducing. a. C. b. HO CH₂OH CH2OH OH OH OH OH OH HOCH₂ OH OH OH HOCH₂ HO HO HO OH OH ΙΟ CH₂OH OH OH "OH OHarrow_forward
- For a red blood cell (erythrocyte) undergoing active glycolysis, briefly explain how increases in concentration of the following factors are likely to affect glycolytic flux. a. ATP b. AMP c. F-1,6-BP d. F-2,6-BP e. Citrate f. Glucose-6-phosphatearrow_forwardThe ∆G°’ for hydrolysis of phosphoenol pyruvate is -62.2 kJ/mol. The standard freeenergy of ATP hydrolysis is -30.5 kJ/mol. A. What is the standard free energy and K eq of the spontaneous reaction betweenADP/ATP and phosphoenol pyruvate. B. Repeat A for F-1,6-BP (∆G°’=-16.7 kJ/mol) and 1,3-BPG (∆G°’=-49.6 kJ/mol)hydrolysis. C. If the ATP and ADP concentrations are 8mM and 1mM respectively, what would bethe ratio of pyruvate/phosphoenolpyruvate at equilibrium?arrow_forwardAnswerarrow_forward
- 13. Which one is the major organic product of the following sequence of reactions? A OH (CH3)2CHCH2COOH SOCI2 CH3OH 1. CH3MgBr 2. H₂O, H+ B C D OH E OHarrow_forward14. Which one is the major organic product of the following sequence of reactions? (CH3)2CH-COCI CH3OH 1. DIBALH, -78°C 1. PhCH2MgBr ? 2. H2O, HCI 2. H2O, HCI OH OMe A Ph B Ph OH Ph C OMe Ph D E OH .Pharrow_forward6. Which one is the major organic product obtained from the following reaction? CO₂Me 1. LiAlH4 2. H₂O CH₂OH CH₂OCH3 5555 HO A B HO C HO D CH₂OH E ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY