
Concept explainers
PRACTICE PROBLEM 15.8
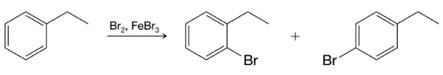
Write resonance structures for the arenium ions formed when ethylbenzene reacts with a
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Chemistry: Structure and Properties (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Organic Chemistry (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)