
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 40P
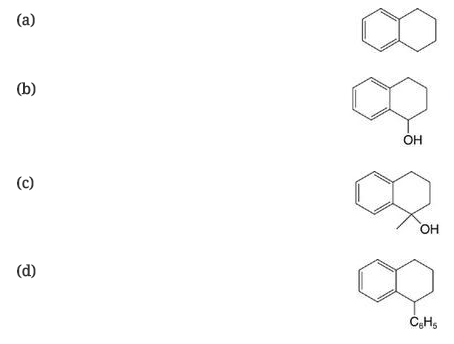
Show how you might synthesize each of the following starting with α-tetralone (Section 15.9 ):
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the options that are true when referring to electrode membranes:1. The Donnan potential, in general, does not always intervene in membranes.2. There are several ways to classify the same membrane.3. Any membrane can be used to determine the pH of a solution.4. Only one solution and one membrane are needed to determine the pH of that solution.
Calculate the maximum volume of carbon dioxide gas
In galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential difference
Chapter 15 Solutions
Organic Chemistry
Ch. 15 - PRACTICE PROBLEM 15.1
Show how loss of a proton...Ch. 15 - Prob. 2PPCh. 15 - PRACTICE PROBLEM 15.3
Outline all steps in a...Ch. 15 - PRACTICE PROBLEM 15.4 Provide a mechanism that...Ch. 15 - Prob. 5PPCh. 15 - PRACTICE PROBLEM
15.6 Explain how the percentages...Ch. 15 - PRACTICE PROBLEM
15.7
Predict the major products...Ch. 15 - Prob. 8PPCh. 15 - Prob. 9PPCh. 15 - Prob. 10PP
Ch. 15 - PRACTICE PROBLEM 15.8 Write resonance structures...Ch. 15 - PRACTICE PROBLEM 15.9
Provide a mechanism for the...Ch. 15 - PRACTICE PROBLEM 15.13
Write mechanisms for the...Ch. 15 - Prob. 14PPCh. 15 - PRACTICE PROBLEM 15.15
Suppose you needed to...Ch. 15 - PRACTICE PROBLEM
15.16 Predict the major product...Ch. 15 - PRACTICE PROBLEM Account for the following...Ch. 15 - PRACTICE PROBLEM 1-Chloro-3-methyl-2-butene...Ch. 15 - Prob. 19PPCh. 15 - PRACTICE PROBLEM
15.20 The following chlorides (Ph...Ch. 15 - Prob. 21PPCh. 15 - Provide a detailed mechanism for each of the...Ch. 15 - 15.34 Provide a detailed mechanism for the...Ch. 15 - Prob. 24PCh. 15 - Many polycyclic aromatic compounds have been...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - Predict the major product (or products) formed...Ch. 15 - Prob. 29PCh. 15 - Prob. 30PCh. 15 - Predict the major products of the following...Ch. 15 - Prob. 32PCh. 15 - Prob. 33PCh. 15 - Prob. 34PCh. 15 - Starting with aniline, outline a synthesis of each...Ch. 15 - Prob. 36PCh. 15 - 15.37 Propose structures for compounds G–I:
Ch. 15 - 2,6-Dichlorophenol has been isolated from the...Ch. 15 - 2-Methylnaphthalene can be synthesized from...Ch. 15 - Show how you might synthesize each of the...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 15.47 Provide structures for compounds A and B:
Ch. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Treating cyclohexene with acetyl chloride and...Ch. 15 - 15.47 The tert-butyl group can be used as a...Ch. 15 - When toluene is sulfonated (concentrated H2SO4) at...Ch. 15 - Prob. 49PCh. 15 - 15.50 Heating 1,1,1-triphenylmethanol with ethanol...Ch. 15 - 15.51
(a) Which of the following halides would you...Ch. 15 - Furan undergoes electrophilic aromatic...Ch. 15 - 15.61 Acetanilide was subjected to the following...Ch. 15 - Prob. 54PCh. 15 - When compound C, which is often used to model a...Ch. 15 - The structure of thyroxine, a thyroid hormone that...Ch. 15 - Prob. 2LGPCh. 15 - 3. Deduce the structures of compounds E–L in the...Ch. 15 - Which of the following compounds would be most...Ch. 15 - 15.2 Which of the following is not a...Ch. 15 - Prob. 3QCh. 15 - Complete the following syntheses.
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Which joints are formed by the femur?
Principles of Anatomy and Physiology
17.25 You are asked to prepare a pH = 3.00 buffer solution starting from 1.25 L of a 1.00 M solution of hydrofl...
Chemistry: The Central Science (14th Edition)
Why can algae and cyanobacteria be considered indicators of productivity as well as of pollution?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Use the key to classify each of the following described tissue types into one of the four major tissue categori...
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forwardRadiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forward
- Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forwardDraw a curved arrow mechanism for its formation. You may need to re-draw structures to show certain bonds. Ensure that HSO is used as the base to deprotonate the ẞ carbon when necessary. C HO : OH HO: OH =s = + 1 Add/Remove step X Click and drag to start drawing a structure.arrow_forwardWhich of the following could 1,2-ethanediol be directly synthesized from? OH HO О 0 0. O ?arrow_forward
- Design a synthesis of 1,2-diethoxyethane from an alkene. Select the single best answer for each part. Part: 0/3 Part 1 of 3 Which of the following could 1,2-diethoxyethane be directly synthesized from? O HO 0 HO.... OH HO HO × 5 > ?arrow_forwardDraw the skeletal structure of the major organic product of each step of the reaction sequence. Part: 0/2 Part 1 of 2 Part: 1/2 Part 2 of 2 Continue OH NaH Na Na Br + Click and drag to start drawing a structure. X : X G : Garrow_forwardpleasearrow_forward
- please help me please pleasearrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N2 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm ☑ 5 00. 18 Ararrow_forwardi need help with the followingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY