
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.4, Problem 5P
How many alkyl chlorides can be obtained from monochlorination of the following


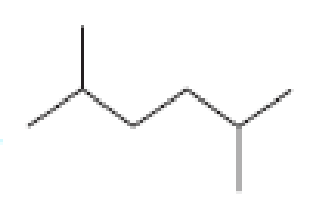
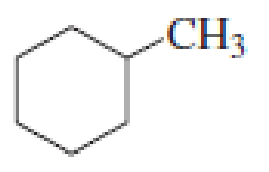
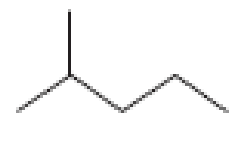
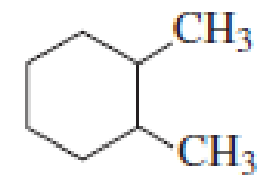
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed
a chemical structure shown below:
% Transmittance
4000
3500
3000
2500 2000
Wavenumber (cm-1)
1500
1000
(a) Explain why her proposed structure is inconsistent with the IR spectrum obtained
(b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure
and explain why it is more compatible with the obtained spectrum.
(C) what is the possible source for the fairly intense signal at
1621cm1
AE>AE₁ (Y/N)
AE=AE₁ (Y/N)
AE
Treatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and
gives compound A.
?
NH2
Br
Br
Propose a structural formula for compound A.
You do not have to explicitly draw H atoms.
You do not have to consider stereochemistry.
In cases where there is more than one answer, just draw one.
R3N
C14H19NO2
+ 2 R3NH*Br
A
Chapter 14 Solutions
Essential Organic Chemistry, Global Edition
Ch. 14.2 - Write the mechanism for the monobromination of...Ch. 14.2 - Prob. 2PCh. 14.4 - Prob. 3PCh. 14.4 - Which of the hydrogens in the structure in the...Ch. 14.4 - How many alkyl chlorides can be obtained from...Ch. 14.4 - Prob. 7PCh. 14.5 - Prob. 9PCh. 14.6 - a. Which ether is most apt to form a peroxide? b....Ch. 14.7 - Prob. 11PCh. 14 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Correctly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forward
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY