
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.4, Problem 3P
Interpretation Introduction
Interpretation:
Number of hydrogen atoms which are attached to the secondary carbons in the given structure has to be determined.
Concept introduction:
In accordance with the bonds and the connection the carbon atom is an organic molecule can be classified into four and they are mentioned below,
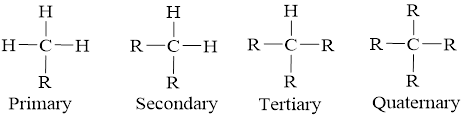
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide reactions showing the following conversions: * see image
. Draw structures corresponding to each of the following names or Provide IUPAC names for each of the
ollowing structures [for 4 ONLY].
A. 2-propylpentanoic acid.
B. m-chlorobenzoic acid.
D.
C.
O
O
HOC(CH2)3COH glutaricadd
OH
OH
H3C
CH3
C=C
H
COOH
salicylicadd
tiglicadd
CH₂C=N
Joe M
. Provide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the
ollowing reactions or sequences of reactions. Show all relevant stereochemistry [five only]
A.
O
B.
OET
CH3
1. LIAIH, ether
2 H₂O
O
(CH3)2CH-C-CI +
0
0
ether (CH3)2CH-C-O-C-CH3
CH3
C.
0
OH
HO
CH3
°
Cl
Chapter 14 Solutions
Essential Organic Chemistry, Global Edition
Ch. 14.2 - Write the mechanism for the monobromination of...Ch. 14.2 - Prob. 2PCh. 14.4 - Prob. 3PCh. 14.4 - Which of the hydrogens in the structure in the...Ch. 14.4 - How many alkyl chlorides can be obtained from...Ch. 14.4 - Prob. 7PCh. 14.5 - Prob. 9PCh. 14.6 - a. Which ether is most apt to form a peroxide? b....Ch. 14.7 - Prob. 11PCh. 14 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How would you prepare each of the following compounds using either an acetoacetic ester synthesis or a alonic ester synthesis? Show all intermediate structures and all reagents.[Three only] A. B. COOH OH C. D. 0 H2C CHCH2CH2CCH3arrow_forwardFats and greases have mostly aliphatic regions which are hydrophobic. Provide a schematic of howsoaps/detergents remove fats and grease from the soiled material. * see imagearrow_forwardWhat chemical has the common name "lye"? Pick one of the 3 esters and show the hydrolysis mechanism to make a carboxylic acid. The organic “R” should be used to limit the redrawing time of the entire molecule. * see imagearrow_forward
- Provide the products for each reaction. There are two and they are not related. *see imagearrow_forwardd. a phenylal Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all levant stereochemistry. [three only] 0 A. B. CH3 Bra CH3COOH OH 1. Br₂, PBrz 2 H₂O 12arrow_forward2arrow_forward
- Show how the following conversions might be accomplished. Show all reagents and all intermediate ructures. More than one step may be required [2 ONLY]: A. B. ° C. OH 0 OH 0arrow_forwardA 20.3 mL sample of 0.263 M triethylamine, (C2H5)3N, is titrated with 0.252 M hydrochloric acid. (1) At the titration midpoint, the pH is . (2) At the equivalence point, the pH is .arrow_forwardd. 3,4,5-trimethoxybenzoyl chloride . What is the order of decreasing reactivity towards nucleophilic acyl substitution for the arboxylic acid derivatives? (most reactive first) A. B. 0 0 O 0 0 H3C-C-O-C-CH3 H3C-C-N(CH3)2 H3C-C-OCH 3 (CH3)2CH-C-OCH3 I || ။ IV a. I, II, III, IV b. I, III, IV, II C. II, IV, III, I d. II, I, III, IV 0 0 0 0 0 R-C-O C-R R-C-NH2 R-C OR R-C-CI a. I, III, II, IV | 11 III IV b. II, III, I, IV c. III, II, I, IV d. IV, I, III, IIarrow_forward
- B. d. a hydrate 4. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [4 ONLY]. A. CH₂OH PCC CH2Cl2 0 H KCN HCN 2arrow_forwardPropose a synthesis of the anti-inflammatory drug Ibuprofen from benzene. Show all reagents and all intermediate structures. Assume that ortho and para isomers can be separated. (CH3)2CHCH2 CH3 CHCOOH 1buprofen be requiredarrow_forwardAssuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product: (a) KHC4H4O6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY