
(a)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
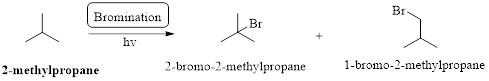
(b)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
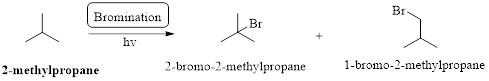
(c)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
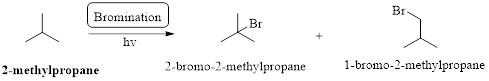
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry, Global Edition
- How would you prepare each of the following compounds using either an acetoacetic ester synthesis or a alonic ester synthesis? Show all intermediate structures and all reagents.[Three only] A. B. COOH OH C. D. 0 H2C CHCH2CH2CCH3arrow_forwardFats and greases have mostly aliphatic regions which are hydrophobic. Provide a schematic of howsoaps/detergents remove fats and grease from the soiled material. * see imagearrow_forwardWhat chemical has the common name "lye"? Pick one of the 3 esters and show the hydrolysis mechanism to make a carboxylic acid. The organic “R” should be used to limit the redrawing time of the entire molecule. * see imagearrow_forward
- Provide the products for each reaction. There are two and they are not related. *see imagearrow_forwardd. a phenylal Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all levant stereochemistry. [three only] 0 A. B. CH3 Bra CH3COOH OH 1. Br₂, PBrz 2 H₂O 12arrow_forward2arrow_forward
- Show how the following conversions might be accomplished. Show all reagents and all intermediate ructures. More than one step may be required [2 ONLY]: A. B. ° C. OH 0 OH 0arrow_forwardA 20.3 mL sample of 0.263 M triethylamine, (C2H5)3N, is titrated with 0.252 M hydrochloric acid. (1) At the titration midpoint, the pH is . (2) At the equivalence point, the pH is .arrow_forwardd. 3,4,5-trimethoxybenzoyl chloride . What is the order of decreasing reactivity towards nucleophilic acyl substitution for the arboxylic acid derivatives? (most reactive first) A. B. 0 0 O 0 0 H3C-C-O-C-CH3 H3C-C-N(CH3)2 H3C-C-OCH 3 (CH3)2CH-C-OCH3 I || ။ IV a. I, II, III, IV b. I, III, IV, II C. II, IV, III, I d. II, I, III, IV 0 0 0 0 0 R-C-O C-R R-C-NH2 R-C OR R-C-CI a. I, III, II, IV | 11 III IV b. II, III, I, IV c. III, II, I, IV d. IV, I, III, IIarrow_forward
- B. d. a hydrate 4. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [4 ONLY]. A. CH₂OH PCC CH2Cl2 0 H KCN HCN 2arrow_forwardPropose a synthesis of the anti-inflammatory drug Ibuprofen from benzene. Show all reagents and all intermediate structures. Assume that ortho and para isomers can be separated. (CH3)2CHCH2 CH3 CHCOOH 1buprofen be requiredarrow_forwardAssuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product: (a) KHC4H4O6arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

