
Concept explainers
(a)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present.(Bromination reactions are more selective reaction).
The mechanism of monobromination of ethane (as an example) includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
Thus the mechanism of monobromination of ethane is shown below,
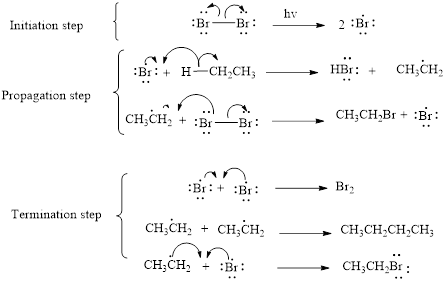
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination will occur on tertiary radical than the secondary than primary radical, tertiary radical is more stable radical than the other radicals.
(a)
Answer to Problem 12P
3-methyl hexane undergoes radical bromination and yields the 3-bromo-3-methylhexane which is shown below
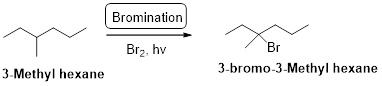
Explanation of Solution
3-methyl hexane undergoes radical bromination and yields the 3-bromo-3-methylhexane according to the above mentioned mechanism steps. The reaction is shown below,
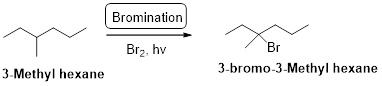
(b)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorination:
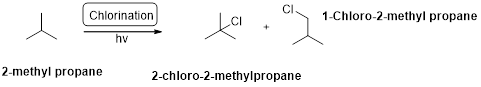
2-methyl propane undergoes radical chlorination and yields the 2-bromo-2-methylpropane and 1-bromo-2-methyl propane.
The mechanism of monochlorination of ethane includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,
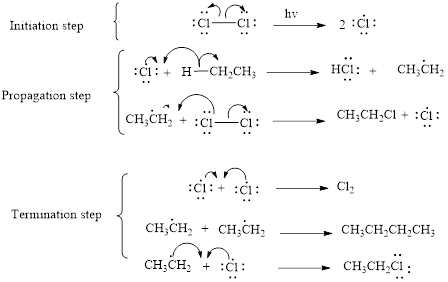
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
(b)
Explanation of Solution
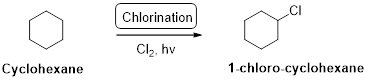
Cyclohexane undergoes radical chlorination; all the carbons in cyclohexane are secondary. Therefore, it yields the 1-chloro cyclohexane according to the above mentioned mechanism steps. And the reaction is shown above.
(c)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorination:
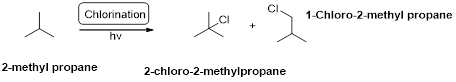
2-methyl propane undergoes radical chlorination and yields the 2-bromo-2-methylpropane and 1-bromo-2-methyl propane.
The mechanism of monochlorination of ethane includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,

In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
(c)
Explanation of Solution
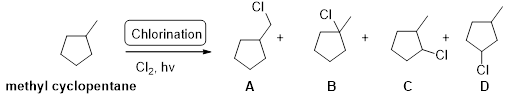
Methyl cyclopentane undergoes radical chlorination; the carbons in cyclopentane are secondary and primary. Therefore, it yields the four types of chlorocyclopentane according to the above mentioned mechanism steps. And the reaction is shown below
Want to see more full solutions like this?
Chapter 14 Solutions
Essential Organic Chemistry, Global Edition
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

