
Concept explainers
(a)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (
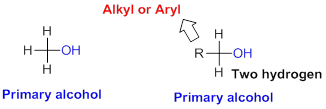
Secondary alcohol:
Hydroxyl group (
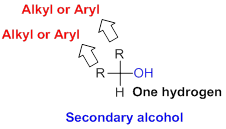
Tertiary alcohol:
Hydroxyl group (
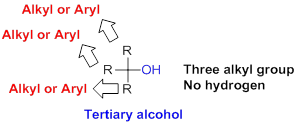
(b)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (
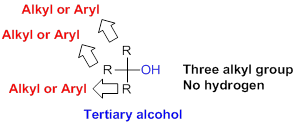
(c)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(d)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(e)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(f)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(g)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(h)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(i)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
- Which features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forwardLooking at the figure 30-5 what intermolecular forces are present between the substrate and the enzyme and the substrate and cofactors.arrow_forwardprovide short answers to the followings Urgent!arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forward
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning  Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning





