
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
8th Edition
ISBN: 9780135213759
Author: John McMurry, David Ballantine
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.10, Problem 14.19P
Interpretation Introduction
Interpretation:
The reason should be explained for 2-aminobutane is chiral.
Concept Introduction:
Chiral:
The carbon atom which attached to the four different atoms is called as chiral. A molecule is non superimposable on its mirror image is called chiral molecule. The molecule is called as chiral molecule, the carbon is called as chiral carbon.
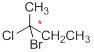
Chiral molecule
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help
You have isolated a protein and determined that the native molecular weight of the holoenzyme is 160 kD using size exclusion chromatography. Analysis of this protein using SDS-PAGE revealed 2 bands, one at 100 kD and one at 30 kD.
Describe the architecture of the polypeptide component of this enzyme.
In a cell free preparation of beta-lactamase, penicillin is hydrolyzed in a D2O enriched assay. After one round of catalysis, where would you anticipate finding Deuterium?
please help thank you
Chapter 14 Solutions
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
Ch. 14.1 - Identify each of the following compounds as an...Ch. 14.1 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - For each of the following molecules, (i) redraw...Ch. 14.4 - Prob. 14.1MRPCh. 14.4 - Provide the mechanism for the dehydration of...Ch. 14.4 - Prob. 14.3MRP
Ch. 14.4 - Prob. 14.8PCh. 14.4 - What alcohols yield the following alkenes as the...Ch. 14.4 - Prob. 14.10KCPCh. 14.4 - What products would you expect from oxidation of...Ch. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13KCPCh. 14.5 - Prob. 14.14PCh. 14.5 - Prob. 14.15PCh. 14.7 - Prob. 14.1CIAPCh. 14.7 - Prob. 14.2CIAPCh. 14.7 - Prob. 14.3CIAPCh. 14.7 - Prob. 14.16PCh. 14.8 - What disulfides would you obtain from oxidation of...Ch. 14.9 - Prob. 14.18PCh. 14.10 - Prob. 14.19PCh. 14.10 - Prob. 14.20PCh. 14.10 - Prob. 14.4CIAPCh. 14.10 - Prob. 14.5CIAPCh. 14.10 - Prob. 14.6CIAPCh. 14.10 - Prob. 14.7CIAPCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - How do alcohols, ethers, and phenols differ...Ch. 14 - What is the structural difference between primary,...Ch. 14 - Prob. 14.28APCh. 14 - Prob. 14.29APCh. 14 - The Taxane nucleus is shown here; it is the basis...Ch. 14 - Vitamin E has the structure shown. Identify the...Ch. 14 - Give systematic names for the following alcohols:...Ch. 14 - Give systematic names for the following compound...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Prob. 14.36APCh. 14 - Locate the alcohol functional groups in the taxane...Ch. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Assume that you have samples of the following two...Ch. 14 - Which of the following alcohols can undergo...Ch. 14 - The following alkenes can be prepared by...Ch. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - What alcohols would you oxidize to obtain the...Ch. 14 - Prob. 14.50APCh. 14 - What is the structural relationship between a...Ch. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Identify the chiral center(s) in each of the...Ch. 14 - Are the following molecules chiral or achiral? If...Ch. 14 - Prob. 14.60CPCh. 14 - Prob. 14.61CPCh. 14 - 1-Propanol is freely soluble in water, 1-butanol...Ch. 14 - Prob. 14.63CPCh. 14 - Prob. 14.64CPCh. 14 - Prob. 14.65CPCh. 14 - Prob. 14.66CPCh. 14 - Prob. 14.67CPCh. 14 - Prob. 14.68CPCh. 14 - Prob. 14.69CPCh. 14 - Prob. 14.70CPCh. 14 - Prob. 14.71CPCh. 14 - Prob. 14.72CPCh. 14 - (a)Draw all possible cyclic C7H14O alcohol isomers...Ch. 14 - Prob. 14.74GPCh. 14 - Prob. 14.75GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- To map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. question: the b-lactamase hydrolyzes the lactam-ring in antibiotics like penicillin. Describe the mechanism, of hydrolysis, insuring to include the involvement of S, D, and K in the reaction sequence. Please help!arrow_forwardThree of these amino acids participate in the proteolytic hydrolysis of polypeptides. Show the charge-relay network generated by the serine proteases and identify the nucleophilic species that initiates the hydrolysis. please help!arrow_forwardYou have isolated a protein and determined that the native molecular weight of the holoenzyme is 160 kD using size exclusion chromatography. Analysis of this protein using SDS-PAGE revealed 2 bands, one at 100 kD and one at 30 kD. 1. Describe the architecture of the polypeptide component of this enzyme. 2. The enzyme was found to be 0.829% NAD (by weight). What further can be said regarding the architecture? can you please help me with question number 2arrow_forward
- To map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Question: although S, K, and D are involved in the catalysis, the E in this hexapeptide does not participate in the hydrolysis of the b-lactam ring. Why is that?arrow_forwardTo map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. a) Using the experimental results described below deduce the primary sequence of the active site hexapeptide. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. please help!arrow_forwardThe beta-lactamase hydrolyzes the lactam-ring in penicillin. Describe the mechanism of hydrolysis, insuring to include the involvement of S, D, & K in the reaction sequence. Please helparrow_forward
- To map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Why doesn't D in this hexapeptide not participate in the hydrolysis of the beta-lactam ring even though S, K, and D are involved in the catalyst?arrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Using the experimental results described above derive the primary sequence of the active site hexapeptide. Please help!arrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
- +NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forwardWhich features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning


Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biodiversity hotspots and functional diversity; Author: Stockholm Resilience Centre TV;https://www.youtube.com/watch?v=Gr_eIsFOKr4;License: Standard Youtube License