
Concept explainers
(a)
Interpretation:
The dehydrated product formed from the following alcohol with
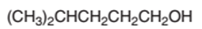
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be a reactant, whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form
Answer to Problem 58P
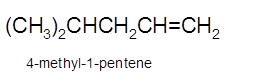
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 4-methylpentanol will form 4-methyl-1-pentene molecule.
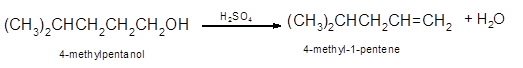
(b)
Interpretation:
The dehydrate product formed from the following alcohol with
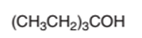
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
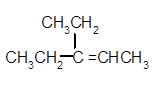
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of given alcohol will form only one alkene as all H next to −OH are the same.
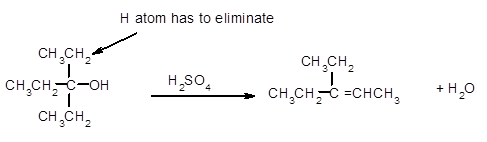
(c)
Interpretation:
The dehydrated product formed from the following alcohol with
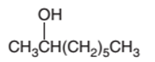
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
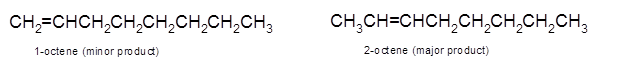
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH groups from two adjacent C's.
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 2-octanol can form two alkenes as both the neighbor C of OH group have H atoms. Hence, dehydration of 2-octanol will follow Zaitsev rule and will form 2-octene as the major product and 1-octene as a minor product as 2-octene is more substituted alkene than 1-octene.
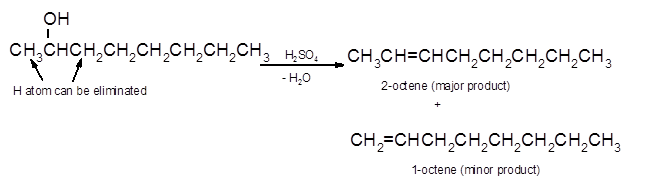
(d)
Interpretation:
The dehydrate product formed from the following alcohol with
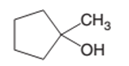
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 58P
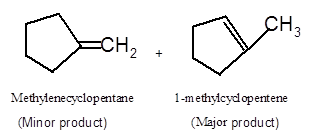
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence the dehydration of given alcohol can form two alkenes as both the neighbor C of OH group have H atoms. Hence dehydration of 1-methylcyclopentanol will follow Zaitsev rule and will form 1-methylcyclpentene as the major product and methylenecyclopentane as a minor product, as 1-methylcyclpentene is more substituted alkene than methylenecyclopentane.
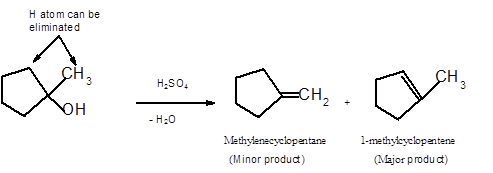
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- '☐ : ☑ ด Suppose an alien life form has DNA just like human DNA remain the same.) - except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled. Note: be sure to draw the molecule as it would exist at physiological pH. Click and drag to start drawing a structure.arrow_forwardPredict the products of the following biochemical reaction: CH2 CH-O + 3 KOH CH2-0 In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. No reaction Click and drag to start drawing a structure. : 5 èarrow_forwardAssign these spectrumarrow_forward
- If I have 30% H2O2, indicate how to prepare a 6% H2O2 solution.arrow_forward7) 8) FCI II -C-C-C=C-C || Br Br || -C=C-Br -CEC-C-C- 10) 11) F Br i OH مله 12) Br i 13) 14) 15) CH3CHFCHFC=CH C(OH)Br2CHF(CH2)4CH2CH3 CH3(CH2)3CH=CH(CH2)2CH3arrow_forwardName 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole





