
(a)
Interpretation: The hybridization for HCN molecule needs to be determined.
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs on each bonded atoms. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms have complete octet.
Hybridization of a molecule can be checked with the help of below formula:
(a)
Answer to Problem 19E
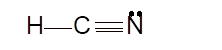
- sp hybridization
Explanation of Solution
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in HCN:
Hence the best Lewis structure for HCN must be:
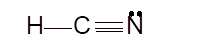
Hybridization of a molecule can be checked with the help of below formula:
(b)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs on each bonded atoms. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms have complete octet.
Hybridization of a molecule can be checked with the help of below formula:
(b)
Answer to Problem 19E
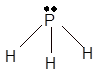
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
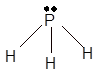
(c)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs on each bonded atoms. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms have complete octet.
Hybridization of a molecule can be checked with the help of below formula:
(c)
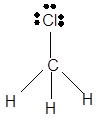
Answer to Problem 19E
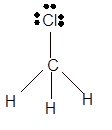
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(d)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(d)
Answer to Problem 19E
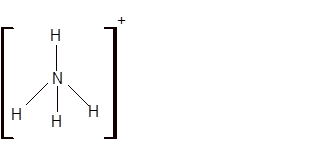
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
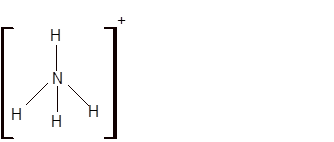
(e)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs on each bonded atoms. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms have complete octet.
Hybridization of a molecule can be checked with the help of below formula:
(e)
Answer to Problem 19E
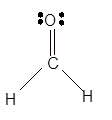
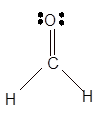
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
(f)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(f)
Answer to Problem 19E
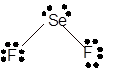
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence, the best Lewis structure for
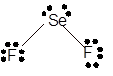
(g)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(g)
Answer to Problem 19E
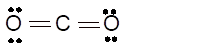
sp- hybridization
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
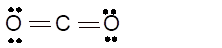
(h)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(h)
Answer to Problem 19E

Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for

(i)
Interpretation: The hybridization of HBr needs to be determined.
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs on each bonded atoms. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms have complete octet.
Hybridization of a molecule can be checked with the help of below formula:
(i)
Answer to Problem 19E

Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for

(j)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(j)
Answer to Problem 19E
Explanation of Solution
To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
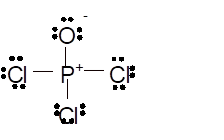
Total number of valence electrons in
Hence the best Lewis structure for
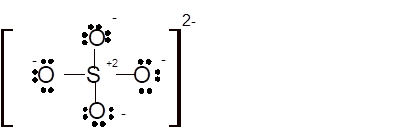
Total number of valence electrons in
Hence the best Lewis structure for
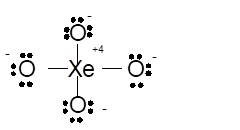
Total number of valence electrons in
Hence the best Lewis structure for
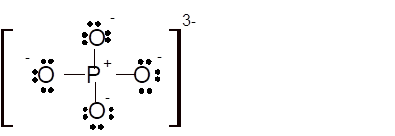
Total number of valence electrons in
Hence the best Lewis structure for
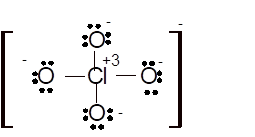
(k)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(k)
Answer to Problem 19E
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
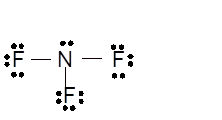
Total number of valence electrons in
Hence the best Lewis structure for
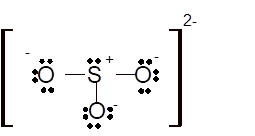
Total number of valence electrons in
Hence the best Lewis structure for
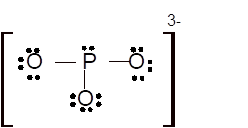
Total number of valence electrons in
Hence the best Lewis structure for
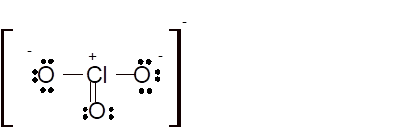
(l)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(l)
Answer to Problem 19E
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
Total number of valence electrons in
Hence the best Lewis structure for
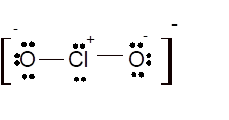
Total number of valence electrons in
Hence the best Lewis structure for
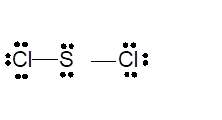
Total number of valence electrons in
Hence the best Lewis structure for
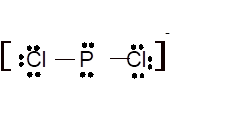
(m)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs present on bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(m)
Answer to Problem 19E
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
According to the given molecular formula, the structure of
Hence the best Lewis structure for

According to the given molecular formula, the structure of
Hence the best Lewis structure for
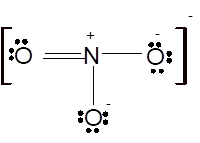
According to the given molecular formula, the structure of
Hence the best Lewis structure for
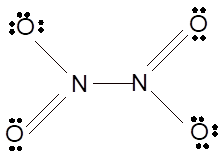
(n)
Interpretation: The hybridization of
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on present on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The sum of valence electrons must be arranged in such a way that all atoms must get octet configuration (8 electrons).
Hybridization of a molecule can be checked with the help of below formula:
(n)
Answer to Problem 19E
Explanation of Solution
The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons. To draw the Lewis structure, calculate the total number of valence electrons in each atom and draw the structure in such a way that each atom gets its octet configuration.
According to the given molecular formula, the structure of
Hence the best Lewis structure for
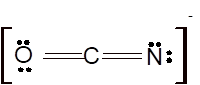
According to the given molecular formula, the structure of
Hence the best Lewis structure for
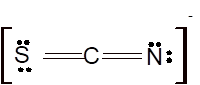
According to the given molecular formula, the structure of
Hence the best Lewis structure for

Want to see more full solutions like this?
Chapter 14 Solutions
EBK CHEMICAL PRINCIPLES
- What is the stepwise mechanism for this reaction?arrow_forward32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forward
- K for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forwardDraw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forward
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forwardCalculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forward
- Plleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





