
a)
Interpretation:
The condensed structural formulas for the products of the following have to be drawn.
Concept Introduction:
➢ The condensed structural formula shows as the atoms that form the molecule are united. The bonds between the carbons and the hydrogen are not shown. The hydrogen united to carbon are represented with its chemical element that has a subscript that represents the amount of hydrogens united to the carbon.
➢ The esters are oil derivatives that are formed when a
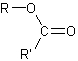
b)
Interpretation:
The condensed structural formulas for the products of the following have to be drawn.
Concept Introduction:
➢ The condensed structural formula shows as the atoms that form the molecule are united. The bonds between the carbons and the hydrogen are not shown. The hydrogen united to carbon are represented with its chemical element that has a subscript that represents the amount of hydrogens united to the carbon.
➢ The esters are oil derivatives that are formed when a carboxylic acid reacts with an alcohol losing a molecule of water. Its general formula is,

c)
Interpretation:
The condensed structural formulas for the products of the following have to be drawn.
Concept Introduction:
➢ The condensed structural formula shows as the atoms that form the molecule are united. The bonds between the carbons and the hydrogen are not shown. The hydrogen united to carbon are represented with its chemical element that has a subscript that represents the amount of hydrogens united to the carbon.
➢ The esters are oil derivatives that are formed when a carboxylic acid reacts with an alcohol losing a molecule of water. Its general formula is,

Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- Predict the product formed when the compound shown below undergoes a reaction with MCPBA in CH2Cl2. MCPBA is meta-chloroperoxybenzoic acid.arrow_forwardk https://app.aktiv.com STARTING AMOUNT 6 58°F Clear + F1 X Dimensional Analysis - Aktiv Chemistry Your Aktiv Learning trial expires on 02/25/25 at 02:14 PM Question 19 of 22 Polyethylene terephthalate (PET) is used in plastic water bottles. A water bottle has a mass of 14.0 grams. Given a density of 1.38 g/cm³, what is the volume of the plastic used to make the water bottle in cm³ ? ADD FACTOR ANSWER RESET ว 100 14.0 0.01 10.1 1000 0.099 1.38 0.001 Q Search F5 -O+ F6 F7 + F3 F2 W E S4 ST #3 F4 % 5 Y R S & 7 cm³ g/cm³ g ם F8 * 00 8 F9 P ل DOD S F10 F11 F12 Insert D F G H J K + 11arrow_forwardA doctor gives a patient 10 Ci of beta radiation. How many betaparticles would the patient receive in 1 minute? (1 Ci = 3.7 x 1010d/s)arrow_forward
- Part C IN H N. Br₂ (2 equiv.) AlBr3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and + e (×) H± 12D T EXP. L CONT. דarrow_forward9. OA. Rank the expected boiling points of the compounds shown below from highest to lowest. Place your answer appropriately in the box. Only the answer in the box will be graded. (3) points) OH OH بر بد بدید 2 3arrow_forwardThere is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS). Ca, ppm V, ppm SCa, arb. units SV, arb. units 20.0 10.0 14375.11 14261.02 40.0 10.0 36182.15 17997.10 60.0 10.0 39275.74 12988.01 80.0 10.0 57530.75 14268.54 100.0…arrow_forward
- A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C. H₂O(g) + C₁₂O(g) = 2 HOCl(g) K = 0.0900 at 25°C с Calculate the equilibrium concentrations of each gas at 25 °C. [H₂O]= [C₁₂O]= [HOCI]= M Σ Marrow_forwardWhat units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





