
a)
Interpretation:
The following compounds have to be classified and their common name has to be determined.
Concept Introduction:
➢ The
➢ The amines can be classified in the following way,
● Primary amine when one hydrogen of ammonia molecule is replaced.
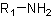
● Secondary amine when two hydrogens of ammonia molecule are replaced.
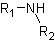
● Tertiary amine when three hydrogens of ammonia molecule are replaced.
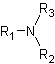
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily
b)
Interpretation:
The following compounds have to be classified and their common name has to be determined.
Concept Introduction:
➢ The amines are ammonia derivatives that are formed when one or more hydrogens of the molecule are replaced by alkyl radicals or other types of substituents.
➢ The amines can be classified in the following way,
● Primary amine when one hydrogen of ammonia molecule is replaced.
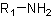
● Secondary amine when two hydrogens of ammonia molecule are replaced.
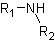
● Tertiary amine when three hydrogens of ammonia molecule are replaced.
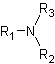
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily
c)
Interpretation:
The following compounds have to be classified and their common name has to be determined.
Concept Introduction:
➢ The amines are ammonia derivatives that are formed when one or more hydrogens of the molecule are replaced by alkyl radicals or other types of substituents.
➢ The amines can be classified in the following way,
● Primary amine when one hydrogen of ammonia molecule is replaced.
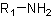
● Secondary amine when two hydrogens of ammonia molecule are replaced.
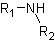
● Tertiary amine when three hydrogens of ammonia molecule are replaced.
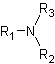
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





