
Interpretation:
The IUPAC name and the common named (if any) for each of the following amides have to be given
Concept Introduction:
➢ The amides are compounds that have the following
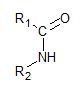
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name of amides is formed of the following way,
1-Write the position of the substituents. If the substituents are in the carbon chain from
2-Write the name of the substituents. They are ordered alphabetically.
3-Write the suffix that indicates the number of carbon that the carbon chain from carboxylic acid has and after that the word -amide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





