
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 14.5, Problem 14.34QAP
Interpretation Introduction
Interpretation:
We have to classify the following
Concept Introduction:
➢ The amines are ammonia derivatives that are formed when one or more hydrogens of the molecule are replaced by alkyl radicals by other types of substituents.
➢ The amines can be classified in the following way,
● Primary amine when one hydrogen of ammonia molecule is replaced.
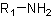
● Secondary amine when two hydrogens of ammonia molecule are replaced.
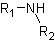
● Tertiary amine when three hydrogens of ammonia molecule are replaced.
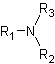
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
McLafferty Rearrangement: Label alpha (), beta (), and gamma () on the molecule. Draw mechanismarrows to describe the process of the rearrangement. What functional group is lost during the rearrangement? What new functional group is made from the ketone/aldehyde you started with? What stabilizing chemical theory causes (allows) rearrangement to happen?
Don't used hand raiting and don't used Ai solution
Don't used hand raiting
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 14.1 - What carboxylic acid is responsible for the pain...Ch. 14.1 - What carboxylic acid is found in vinegar?Ch. 14.1 - Prob. 14.3QAPCh. 14.1 - Prob. 14.4QAPCh. 14.1 - Prob. 14.5QAPCh. 14.1 - Prob. 14.6QAPCh. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.2 - Identify the compound in each group that is most...Ch. 14.2 - Prob. 14.10QAP
Ch. 14.2 - Prob. 14.11QAPCh. 14.2 - Prob. 14.12QAPCh. 14.2 - Prob. 14.13QAPCh. 14.2 - Prob. 14.14QAPCh. 14.3 - Prob. 14.15QAPCh. 14.3 - Prob. 14.16QAPCh. 14.3 - Prob. 14.17QAPCh. 14.3 - Prob. 14.18QAPCh. 14.3 - Prob. 14.19QAPCh. 14.3 - Prob. 14.20QAPCh. 14.3 - Prob. 14.21QAPCh. 14.3 - Prob. 14.22QAPCh. 14.3 - Prob. 14.23QAPCh. 14.3 - Prob. 14.24QAPCh. 14.4 - What are the products of the acid hydrolysis of an...Ch. 14.4 - Prob. 14.26QAPCh. 14.4 - Prob. 14.27QAPCh. 14.4 - Prob. 14.28QAPCh. 14.5 - Prob. 14.29QAPCh. 14.5 - Prob. 14.30QAPCh. 14.5 - Prob. 14.31QAPCh. 14.5 - Prob. 14.32QAPCh. 14.5 - Prob. 14.33QAPCh. 14.5 - Prob. 14.34QAPCh. 14.5 - Prob. 14.35QAPCh. 14.5 - Prob. 14.36QAPCh. 14.5 - Prob. 14.37QAPCh. 14.5 - Prob. 14.38QAPCh. 14.6 - Prob. 14.39QAPCh. 14.6 - Prob. 14.40QAPCh. 14.6 - Prob. 14.41QAPCh. 14.6 - Prob. 14.42QAPCh. 14.6 - Prob. 14.43QAPCh. 14.6 - Prob. 14.44QAPCh. 14.6 - Prob. 14.45QAPCh. 14.6 - Prob. 14.46QAPCh. 14 - Prob. 14.47UTCCh. 14 - Prob. 14.48UTCCh. 14 - The ester methyl butanoate has the odor and flavor...Ch. 14 - Prob. 14.50UTCCh. 14 - Prob. 14.51UTCCh. 14 - Melatonin is a naturally occurring compound in...Ch. 14 - Prob. 14.53UTCCh. 14 - Prob. 14.54UTCCh. 14 - Prob. 14.55AQAPCh. 14 - Prob. 14.56AQAPCh. 14 - Prob. 14.57AQAPCh. 14 - Prob. 14.58AQAPCh. 14 - Prob. 14.59AQAPCh. 14 - Prob. 14.60AQAPCh. 14 - Prob. 14.61AQAPCh. 14 - Prob. 14.62AQAPCh. 14 - Prob. 14.63AQAPCh. 14 - Prob. 14.64AQAPCh. 14 - Prob. 14.65AQAPCh. 14 - Prob. 14.66AQAPCh. 14 - Prob. 14.67AQAPCh. 14 - Prob. 14.68AQAPCh. 14 - Prob. 14.69AQAPCh. 14 - Prob. 14.70AQAPCh. 14 - Prob. 14.71AQAPCh. 14 - Prob. 14.72AQAPCh. 14 - Prob. 14.73AQAPCh. 14 - Prob. 14.74AQAPCh. 14 - Prob. 14.75CQCh. 14 - Prob. 14.76CQCh. 14 - Prob. 14.77CQCh. 14 - Prob. 14.78CQ
Knowledge Booster
Similar questions
- If a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forwardO Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forwardc) + H₂Oarrow_forward
- 으 b) + BF. 3 H2Oarrow_forwardQ4: Draw the product of each Lewis acid-bas reaction. Label the electrophile and nucleophile. b) S + AICI 3 + BF 3arrow_forwardQ1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forward
- Determine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forwardUse the following data for an unknown gas at 300 K to determine the molecular mass of the gas.arrow_forward2. Provide a complete retrosynthetic analysis and a complete forward synthetic scheme to make the following target molecule from the given starting material. You may use any other reagents necessary. Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY