
Organic Chemistry As a Second Language: First Semester Topics
4th Edition
ISBN: 9781119110668
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.6, Problem 13.43P
Interpretation Introduction
Interpretation:
How Grignard reaction can be used to prepare the given compound has to be shown.
Concept Introduction:
When a ketone or an aldehyde is attacked by a Grignard reagent, an alcohol is formed and an example for this reaction is given below,
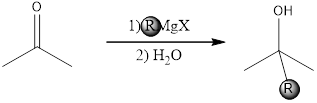
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.
Indicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.
In organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?
Chapter 13 Solutions
Organic Chemistry As a Second Language: First Semester Topics
Ch. 13.1 - Prob. 13.2PCh. 13.1 - Prob. 13.3PCh. 13.1 - Prob. 13.4PCh. 13.1 - Prob. 13.5PCh. 13.2 - Prob. 13.7PCh. 13.2 - Prob. 13.8PCh. 13.2 - Prob. 13.9PCh. 13.2 - Prob. 13.10PCh. 13.3 - PROBLEMS For each pair of compounds, identify the...Ch. 13.3 - Prob. 13.13P
Ch. 13.3 - Prob. 13.14PCh. 13.3 - Prob. 13.15PCh. 13.3 - Prob. 13.16PCh. 13.3 - Prob. 13.17PCh. 13.4 - Prob. 13.19PCh. 13.4 - Prob. 13.20PCh. 13.4 - Prob. 13.21PCh. 13.4 - Prob. 13.22PCh. 13.5 - Prob. 13.24PCh. 13.5 - Prob. 13.25PCh. 13.5 - Prob. 13.26PCh. 13.5 - Prob. 13.27PCh. 13.5 - Prob. 13.28PCh. 13.5 - Prob. 13.29PCh. 13.5 - Prob. 13.31PCh. 13.5 - Prob. 13.32PCh. 13.5 - Prob. 13.33PCh. 13.5 - Prob. 13.34PCh. 13.5 - Prob. 13.35PCh. 13.5 - Prob. 13.36PCh. 13.6 - Prob. 13.38PCh. 13.6 - Prob. 13.39PCh. 13.6 - Prob. 13.40PCh. 13.6 - Prob. 13.41PCh. 13.6 - Prob. 13.42PCh. 13.6 - Prob. 13.43PCh. 13.6 - Prob. 13.44PCh. 13.6 - Prob. 13.45PCh. 13.6 - Prob. 13.46PCh. 13.6 - Prob. 13.47PCh. 13.6 - Prob. 13.48PCh. 13.6 - Prob. 13.49PCh. 13.7 - Prob. 13.51PCh. 13.7 - Prob. 13.52PCh. 13.7 - Prob. 13.53PCh. 13.7 - Prob. 13.54PCh. 13.7 - Prob. 13.55PCh. 13.7 - Prob. 13.56PCh. 13.8 - Prob. 13.58PCh. 13.8 - Prob. 13.59PCh. 13.8 - Prob. 13.60PCh. 13.8 - Prob. 13.61PCh. 13.9 - Prob. 13.63PCh. 13.9 - Prob. 13.64PCh. 13.9 - Prob. 13.65PCh. 13.9 - Prob. 13.66PCh. 13.9 - Prob. 13.67PCh. 13.9 - Prob. 13.68PCh. 13.10 - Prob. 13.70PCh. 13.10 - Prob. 13.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forwardConsider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forwardExplain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.arrow_forward
- Draw the products of a reaction of the following alkyle chloride, shown below in the 3D ball and stick model with NaSCH3. Ignore inorganic byproducts. In the figure, a gray ball indicates a carbon atom a white ball indicates a hydrogen atom anda agreen ball indicated a chlorine atomarrow_forwardDraw the most stable cations formed in the mass spectrometer by a deavage of the following compound Draw the most stable cations formed in the mass spectrometer by a cleavage of the following compound онarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting anand product sytucutrs, draw the curved electron-pusing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bind-making stepsarrow_forward
- Draw the major elimination and substitution products formed in this reavtion. Use a dash or wedge bond to indicatr the stereochemistry of substituents on assymetric centers, wheere applicable. Ignore any inorganic byproducts.arrow_forwardDraw the two possible products produced in this E2 elimination. Ignore any inorganic byproductsarrow_forwardDraw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forward
- Draw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows THE Problem 33 of 35 N. C:0 Na + Submit Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore and inorganic byproducts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY