
Organic Chemistry As a Second Language: First Semester Topics
4th Edition
ISBN: 9781119110668
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.5, Problem 13.33P
Interpretation Introduction
Interpretation:
The starting
Concept Introduction:
Oxidation state of an element is the total number of valence electron that it loses or gains to attain the stable octet structure.
If the oxidation state of the carbon atom has increased after the reaction, then we say oxidation reaction has occurred. A simple example is conversion of an alcohol to ketone/aldehyde or
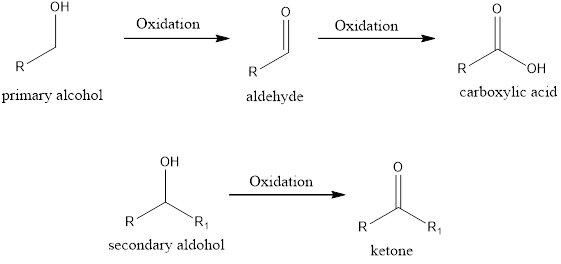
If the oxidation state of the carbon atom has decreased after the reaction, then we say reduction reaction has occurred. A simple example is conversion of ketone/aldehyde to an alcohol.
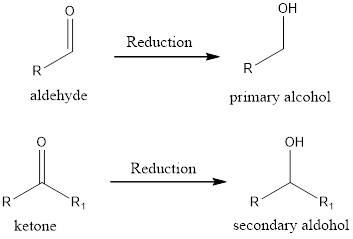
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.
Indicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.
In organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?
Chapter 13 Solutions
Organic Chemistry As a Second Language: First Semester Topics
Ch. 13.1 - Prob. 13.2PCh. 13.1 - Prob. 13.3PCh. 13.1 - Prob. 13.4PCh. 13.1 - Prob. 13.5PCh. 13.2 - Prob. 13.7PCh. 13.2 - Prob. 13.8PCh. 13.2 - Prob. 13.9PCh. 13.2 - Prob. 13.10PCh. 13.3 - PROBLEMS For each pair of compounds, identify the...Ch. 13.3 - Prob. 13.13P
Ch. 13.3 - Prob. 13.14PCh. 13.3 - Prob. 13.15PCh. 13.3 - Prob. 13.16PCh. 13.3 - Prob. 13.17PCh. 13.4 - Prob. 13.19PCh. 13.4 - Prob. 13.20PCh. 13.4 - Prob. 13.21PCh. 13.4 - Prob. 13.22PCh. 13.5 - Prob. 13.24PCh. 13.5 - Prob. 13.25PCh. 13.5 - Prob. 13.26PCh. 13.5 - Prob. 13.27PCh. 13.5 - Prob. 13.28PCh. 13.5 - Prob. 13.29PCh. 13.5 - Prob. 13.31PCh. 13.5 - Prob. 13.32PCh. 13.5 - Prob. 13.33PCh. 13.5 - Prob. 13.34PCh. 13.5 - Prob. 13.35PCh. 13.5 - Prob. 13.36PCh. 13.6 - Prob. 13.38PCh. 13.6 - Prob. 13.39PCh. 13.6 - Prob. 13.40PCh. 13.6 - Prob. 13.41PCh. 13.6 - Prob. 13.42PCh. 13.6 - Prob. 13.43PCh. 13.6 - Prob. 13.44PCh. 13.6 - Prob. 13.45PCh. 13.6 - Prob. 13.46PCh. 13.6 - Prob. 13.47PCh. 13.6 - Prob. 13.48PCh. 13.6 - Prob. 13.49PCh. 13.7 - Prob. 13.51PCh. 13.7 - Prob. 13.52PCh. 13.7 - Prob. 13.53PCh. 13.7 - Prob. 13.54PCh. 13.7 - Prob. 13.55PCh. 13.7 - Prob. 13.56PCh. 13.8 - Prob. 13.58PCh. 13.8 - Prob. 13.59PCh. 13.8 - Prob. 13.60PCh. 13.8 - Prob. 13.61PCh. 13.9 - Prob. 13.63PCh. 13.9 - Prob. 13.64PCh. 13.9 - Prob. 13.65PCh. 13.9 - Prob. 13.66PCh. 13.9 - Prob. 13.67PCh. 13.9 - Prob. 13.68PCh. 13.10 - Prob. 13.70PCh. 13.10 - Prob. 13.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forwardConsider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forwardExplain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.arrow_forward
- Draw the products of a reaction of the following alkyle chloride, shown below in the 3D ball and stick model with NaSCH3. Ignore inorganic byproducts. In the figure, a gray ball indicates a carbon atom a white ball indicates a hydrogen atom anda agreen ball indicated a chlorine atomarrow_forwardDraw the most stable cations formed in the mass spectrometer by a deavage of the following compound Draw the most stable cations formed in the mass spectrometer by a cleavage of the following compound онarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting anand product sytucutrs, draw the curved electron-pusing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bind-making stepsarrow_forward
- Draw the major elimination and substitution products formed in this reavtion. Use a dash or wedge bond to indicatr the stereochemistry of substituents on assymetric centers, wheere applicable. Ignore any inorganic byproducts.arrow_forwardDraw the two possible products produced in this E2 elimination. Ignore any inorganic byproductsarrow_forwardDraw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forward
- Draw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows THE Problem 33 of 35 N. C:0 Na + Submit Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore and inorganic byproducts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY