
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
9th Edition
ISBN: 9781260048636
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.3, Problem 58P
An insulated rigid tank is divided into two compartments by a partition. One compartment contains 7 kg of oxygen gas at 40°C and 100 kPa, and the other compartment contains 4 kg of nitrogen gas at 20°C and 150 kPa. Now the partition is removed, and the two gases are allowed to mix. Determine (a) the mixture temperature and (b) the mixture pressure after equilibrium has been established.
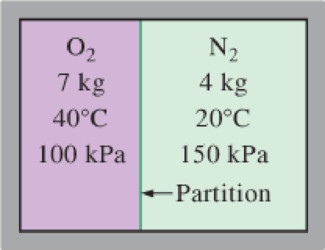
FIGURE P13–58
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A rigid tank that contains 5.2 kg of N2 at 25°C and 550 kPa is connected to another rigid tank that contains 7.2 kg of O2 at 25°C and
150 kPa. The valve connecting the two tanks is opened, and the two gases are allowed to mix. If the final mixture temperature is 25°C,
determine the volume of each tank and the final mixture pressure. The gas constants of N2 and O2 are 0.2968 and
0.2598 kPa-m³/kg-K, respectively. The universal gas constant is 8.314 kPa-m³/kmol-K. Use the table containing the molar mass, gas
constant, and critical-point properties.
N₂
25°C
550 kPa
The volume of the N2 tank is
The volume of the O2 tank is
The final mixture pressure is
m3
Im³
kPa.
0₂
25°C
150 kPa
A mixture of 5 kg of Hydrogen and 26
kg of Nitrogen are contained in a
piston cylinder assembly at a pressure
of 6.78 MPa and a temperature of 125
K. heat is transferred to the device and
the mixture expands at a constant
pressure until the temperature rises to
135 K. Determine the heat transfer in
kJ during the process by treating the
mixture as a non-ideal gas and using
the Amagat's law.
An insulated rigid tank is divided into two compartments by a partition. One compartment contains 7 kg of oxygen gas at 40°C and 100kPa, and the other compartment contains 4 kg of nitrogen gas at 20°C and 150kPa. Now the partition is removed, and the two gases are allowed to mix. Determine:- (a) the mixture temperature and (b) the mixture pressure after equilibrium. CvN2=0.743 kJ/kg K and CvO2 = 0.658 kJ/kg K .
Chapter 13 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
Ch. 13.3 - What are mass and mole fractions?Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 7PCh. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - A gas mixture consists of 20 percent O2, 30...Ch. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - Prob. 18PCh. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Prob. 22PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Prob. 24PCh. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - A gas mixture at 300 K and 200 kPa consists of 1...Ch. 13.3 - Prob. 29PCh. 13.3 - Separation units often use membranes, absorbers,...Ch. 13.3 - Prob. 31PCh. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - An engineer has proposed mixing extra oxygen with...Ch. 13.3 - A rigid tank contains 0.5 kmol of Ar and 2 kmol of...Ch. 13.3 - A mixture of gases consists of 0.9 kg of oxygen,...Ch. 13.3 - Prob. 37PCh. 13.3 - One pound-mass of a gas whose density is 0.001...Ch. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 43PCh. 13.3 - Prob. 44PCh. 13.3 - Prob. 45PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 47PCh. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - Prob. 50PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - A mixture of nitrogen and carbon dioxide has a...Ch. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - A mixture of gases consists of 0.1 kg of oxygen, 1...Ch. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - An insulated rigid tank is divided into two...Ch. 13.3 - Prob. 59PCh. 13.3 - A mixture of 65 percent N2 and 35 percent CO2...Ch. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 68PCh. 13.3 - Prob. 69PCh. 13.3 - The gas passing through the turbine of a simple...Ch. 13.3 - Prob. 71PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 79PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Is it possible for an adiabatic liquid-vapor...Ch. 13.3 - Prob. 84PCh. 13.3 - Prob. 85RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - A mixture of gases is assembled by first filling...Ch. 13.3 - Prob. 90RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 95RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - Prob. 101RPCh. 13.3 - Prob. 102FEPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 105FEPCh. 13.3 - Prob. 106FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEPCh. 13.3 - Prob. 111FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A piston–cylinder device contains 6 kg of H2 and 21 kg of N2 at 160 K and 5 MPa. Heat is now transferred to the device, and the mixture expands at constant pressure until the temperature rises to 200 K. Determine the heat transfer during this process by treating the mixture as a nonideal gas and using Amagat’s law. The universal gas constant is Ru = 8.314 kPa·m3/kmol·K. Use the table containing the molar mass, gas constant, and critical-point properties; the generalized enthalpy departure chart; and the table containing the ideal-gas properties of air.arrow_forwardA mixture of gases is assembled by first filling an evacuated 0.39-m3 tank with neon until the pressure is 35 kPa. Oxygen is added next until the pressure increases to 105 kPa. Finally, nitrogen is added until the pressure increases to 140 kPa. During each step of the tank’s filling, the contents are maintained at 60°C. Determine the mass of each constituent in the resulting mixture. The mass of neon is kg. The mass of oxygen is kg. The mass of nitrogen is kg.arrow_forwardA mixture of nitrogen and carbon dioxide has a carbon dioxide mass fraction of 50 percent. This mixture is heated at constant pressure in a closed system from 120 kPa and 30°C to 220°C. Calculate the work produced during this heating in kJ/kg. The universal gas constant is Ru= 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties. The work produced during this heating is kJ/kg.arrow_forward
- An isolated piston inside a rigid, isolated cylinder, closed at both ends, has two chambers; one containing a two-phase water mixture and one containing a two-phase mixture of R-134a. If the temperature inside the chamber containing water is 85.9°C, determine the temperature inside the other chamber. Assume mechanical equilibrium. Do you have enough data to calculate the quality in both cases? Justify your answer.arrow_forwardTank A which is completely insulated contains 1 kg of oxygen at 15°C and 300 kPa. It is connected to the 2-m3 tanks B through a valve as shown below. Tank B is not insulated and contains nitrogen at 50°C and 500 kPa. An engineer opens the valve connecting the two tanks to allow the two gauges to mix. Once the valve is opened, oxygen and nitrogen form a homogeneous mixture at 25°C. Determine (a) the final pressure in the tank, (b) the heat transfer from tank B, and (c) the entropy change in the system during this process.arrow_forwarduestion 4: (a) An 88-litre gas cylinder is filled with propane gas at a pressure of 1.15 MPa and 18°C. The propane is used to fuel a gas burner. After some time, the pressure and temperature are 210 kPa and 23°C respectively. Determine the mass of propane used. The molar mass of propane is 44 g/mole. (b) A piston-cylinder device filled with air at 365 kPa and 12°C, has an initial volume of 1.3 litres. The air is expanded at constant pressure to a volume of 3.6 litres and 516°C. Determine the amount of heat and work involved in this process and state whether the heat and work are into, or out of the gas.arrow_forward
- A pipe fitted with a closed valve connects two tanks. One tank contains a 5-kg mixture of 62.5 percent CO2 and 37.5 percent O2 on a mole basis at 30°C and 125 kPa. The second tank contains 10 kg of N2 at 15°C and 200 kPa. The valve in the pipe is opened and the gases are allowed to mix. During the mixing process 100 kJ of heat energy is supplied to the combined tanks. The temperature of the mixture after mixing process is 39.4oC. Draw the schematic diagram of the system and all the data displayed on it. List all the appropriate assumption before analyzing the problem. Use subscript ‘m’ to designate mixture. After mixing process, determine (a) the total volume of the mixture and (b) the final pressure of the mixture. Consult the appropriate property tables for the molar masses and gas constants of the constituent gases from the separate PDF file provided on the portal.arrow_forwardThermodynamics question Using PV=mRT and PV=NRuTarrow_forwardA closed rigid vessel contains 40% liquid water and 60% water vapor by volume. The liquid-vapor mixture is in equilibrium at 150°C. What is the quality of the mixture?arrow_forward
- A mixture of hydrocarbon gases is composed of 60 percent methane, 25 percent propane, and 15 percent butane by weight. This mixture is compressed from 100 kPa and 20°C to 1400 kPa in a reversible, isothermal, steady-flow compressor. Calculate the work and heat transfer for this compression per unit mass of the mixture. The universal gas constant is R₁ = 8.314 kPa-m³/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties. P₂ 60% CH4 25% C₂H₂ 15% C₂H10 (by mass) 100 kPa 20°C W The work input for this compression per unit mass of the mixture is The heat transfer for this compression per unit mass of the mixture is kJ/kg. kJ/kg.arrow_forwardA spring-loaded piston–cylinder device contains a mixture of gases whose pressure fractions are 25 percent Ne, 50 percent O2, and 25 percent N2. The piston diameter and spring are selected for this device such that the volume is 0.1 m3 when the pressure is 200 kPa and 1.0 m3 when the pressure is 1000 kPa. Initially, the gas is added to this device until the pressure is 200 kPa and the temperature is 10°C. The device is now heated until the pressure is 550 kPa. Calculate the total work and heat transfer for this process. The universal gas constant is Ru = 8.314 kJ/kmol·K. Use the table containing the ideal-gas specific heats of various common gases and the table containing the molar mass, gas constant, and critical-point properties. The total work for this process is _________kJ. The heat transfer for this process is __________kJ.arrow_forwardOn a hot summer day, you decide to make some iced tea. First, you brew 1.50 L of hot tea and leave it to steep until it has reached a temperature of Ttea = 75.0 ∘C. You then add 0.975 kg of ice taken from the freezer at a temperature of Tice = 0 ∘C. By the time the mix reaches equilibrium, all of the ice has melted. What is the final temperature Tf of the mixture? For the purposes of this problem, assume that the tea has the same thermodynamic properties as plain water. The specific heat of water is c = 4190 J/kg⋅∘C The heat of fusion of ice is Lf = 3.33×105 J/kg The density of the tea is ρ tea = 1.00 kg/Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY