
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
9th Edition
ISBN: 9781260048636
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.3, Problem 52P
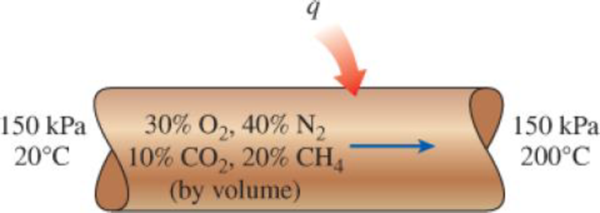
The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. This mixture is heated from 20°C to 200°C while flowing through a tube in which the pressure is maintained at 150 kPa. Determine the heat transfer to the mixture per unit mass of the mixture.
FIGURE P13–52
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A tensile specimen made of hot-rolled AISI 1020 steel is loaded to point corresponding to a strain of 43%.
60
Su = 66 ksi
Stress σ (ksi)
40 B
20
0
0
0
T
H
Sy = 39 ksi
Se = 36 ksi
Hot-rolled 1020 steel
F
10 20 30 40
50 60 70 80 90 100 110 120 130 140 150 160
Strain € (%)
T
1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6
Area ratio R
0.1
0.2
0.3
0.4
0.5
Area reduction A,
What value of strain is applicable to this location?
0.6
A tensile specimen made of hot-rolled AISI 1020 steel is loaded to point corresponding to a strain of 40%.
60
Su = 66 ksi
Stress σ (ksi)
S₁ = 39 ksi
40
Se = 36 ksi
Hot-rolled 1020 steel
20
0
10 20 30 40
50 60 70 80 90 100 110 120 130 140 150 160
Strain € (%)
0
1.1 1.2 1.3 1.4 1.5
1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6
Area ratio R
0.1
0.2
0.3
0.4
0.5
Area reduction A,
What value of area ratio is applicable to this location?
0.6
A tensile specimen made of hot-rolled AISI 1020 steel is loaded to point corresponding to a strain of 43%.
60
Su = 66 ksi
Stress σ (ksi)
20
Sy = 39 ksi
Se = 36 ksi
Hot-rolled 1020 steel
F
0
10 20 30
40 50 60
70 80 90 100 110 120 130 140 150 160
Strain € (%)
0
1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6
Area ratio R
0.1
0.2
0.3
0.4
0.5
Area reduction A,
What value of area reduction is applicable to this location?
0.6
Chapter 13 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
Ch. 13.3 - What are mass and mole fractions?Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 7PCh. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - A gas mixture consists of 20 percent O2, 30...Ch. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - Prob. 18PCh. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Prob. 22PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Prob. 24PCh. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - A gas mixture at 300 K and 200 kPa consists of 1...Ch. 13.3 - Prob. 29PCh. 13.3 - Separation units often use membranes, absorbers,...Ch. 13.3 - Prob. 31PCh. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - An engineer has proposed mixing extra oxygen with...Ch. 13.3 - A rigid tank contains 0.5 kmol of Ar and 2 kmol of...Ch. 13.3 - A mixture of gases consists of 0.9 kg of oxygen,...Ch. 13.3 - Prob. 37PCh. 13.3 - One pound-mass of a gas whose density is 0.001...Ch. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 43PCh. 13.3 - Prob. 44PCh. 13.3 - Prob. 45PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 47PCh. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - Prob. 50PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - A mixture of nitrogen and carbon dioxide has a...Ch. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - A mixture of gases consists of 0.1 kg of oxygen, 1...Ch. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - An insulated rigid tank is divided into two...Ch. 13.3 - Prob. 59PCh. 13.3 - A mixture of 65 percent N2 and 35 percent CO2...Ch. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 68PCh. 13.3 - Prob. 69PCh. 13.3 - The gas passing through the turbine of a simple...Ch. 13.3 - Prob. 71PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 79PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Is it possible for an adiabatic liquid-vapor...Ch. 13.3 - Prob. 84PCh. 13.3 - Prob. 85RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - A mixture of gases is assembled by first filling...Ch. 13.3 - Prob. 90RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 95RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - Prob. 101RPCh. 13.3 - Prob. 102FEPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 105FEPCh. 13.3 - Prob. 106FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEPCh. 13.3 - Prob. 111FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Table of Measurements and Results: Reading m/s Ji- a (wh Nu h Re Nu Error% (C) (°C) 2 1 Discussion: 1-Estimate the heat transfer and experimental value of the heat transfer coefficient hex with its unit and Nusselt number Nu expl 2- Find the percentage error for the value of the experimental Nusselt number. 3-Draw the graph showing a relationship between the temperatures difference (T-T) and theoretical and experimental value of Nusselt number. 4-The forced convection heat transfer coefficient of a plate depends on which of the following: a-gravity. b-velocity of fluid. e-conductivity of fluid. d-conductivity of plate material. Experiment: Internal Forced convenction Heat trovate on now through t objectives. Study the convection heat transfer of air flow through stage Calculations. Q & (T-T) Vary Re Q. heup A (TT) (T. Te-T ASPL Nep Re 117 RITT 14 ' 14arrow_forwardIf AE = 1.6 m, ED = CD = 1.9 m and F = 3.1 kN, then find the magnitude of the force acting in EB. B 30° 30° C E D ED m DC m ♥F KNarrow_forwardAssume multiple single degree of freedom systems with natural periods T ∈ [0.05, 2.00] seconds with in- crement of period dT = 0.05 seconds. Assume three cases of damping ratio: Case (A) ξ = 0%; Case (B) ξ = 2%; Case (C) ξ = 5%. The systems are initially at rest. Thus, the initial conditions are u(t = 0) = 0 and ̇u(t = 0) = 0. The systems are subjected to the base acceleration that was provided in the ElCentro.txt file (i.e., first column). For the systems in Case (A), Case (B), and Case (C) and for each natural period compute the peak acceleration, peak velocity, and peak displacement responses to the given base excitation. Please, use the Newmark method for β = 1/4 (average acceleration) to compute the responses. Create three plots with three lines in each plot. The first plot will have the peak accelerations in y-axis and the natural period of the system in x-axis. The second plot will have the peak velocities in y-axis and the natural period of the system in x-axis. The third plot…arrow_forward
- Determine the resultant stress at points P and Q.arrow_forwardFor the notched specimen with h = 0.13 m and r =11 mm, calculate the nominal stress for F=5 kN. F h F 25 mm Please submit your answer in the units of MPa.arrow_forwardA tensile specimen made of hot-rolled AISI 1020 steel is loaded to point corresponding to a strain of 49%. 60 Su = 66 ksi Stress σ (ksi) Sy = 39 ksi 400B Se = 36 ksi Hot-rolled 1020 steel 20 F 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 Strain € (%) 0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Area ratio R 0.1 0.2 0.3 0.4 0.5 Area reduction A, What value of Su is applicable to this location? 0.6arrow_forward
- A tensile specimen made of hot-rolled AISI 1020 steel is loaded to point corresponding to a strain of 40%. 60 Su = 66 ksi Stress σ (ksi) 40 20 Sy= = 39 ksi Se = 36 ksi Hot-rolled 1020 steel F | G | H 0 10 20 30 40 50 60 0 70 80 90 100 110 120 130 140 150 160 Strain € (%) ☐ T 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Area ratio R 0.1 0.2 0.3 0.4 0.5 Area reduction A, What value of Sy is applicable to this location? 0.6arrow_forwardA vertical .2m by .2m square plate is exposed to saturated water vapor at atmospheric pressure. If the surface temperature is 80 degrees C and the flow is laminar, estimate the loal heat transfer coefficents at the middles and at the bottom of the plate.arrow_forwardA transformer that is 10 cm long, 6.2 cm wide, and 5 cm high is to be cooled by attaching a 10 cm by 6.2 cm wide polished aluminum heat sink(emissivity=.03) to its top surface. The heat sink has seven fins, which are 5 mm high, 2mm thick, and 10 cm long. A fan blows air at 25 degrees C parallel to the passages between the fins. The heat sink is to dissipate 12W of heat, and the base temp of the ehat sink is not to exceed 60 degrees C. Assuming the fins and the base plate to be nearly isothermal and the radiation heat transfer to be negligible, determine the minimum free-stream velocity the fan needs to supply to avoid overheating. Assume the flow is laminar over the entire finned surface of the transformer.arrow_forward
- I need a mechanical engineering expert to solve this question,no Ai pleasearrow_forwardCan you give me the meaning of Combination spanner and Give Examples of Spannersarrow_forwardHW8 A shaft fitted with a flywheel rotates at 650 r.p.m. and drives a machine. The torque of machine varies in a cyclic manner over a period of 2 revolutions. The torque rises from 650 N-m to 2200 N-m uniformly during 110° and remains constant for the following 270°. It then falls uniformly to 600 N-m during the next 100° and remains constant for the end cycle, the cycle being repeated thereafter. Determine the power required to drive the machine and percentage fluctuation in speed, if the driving torque applied to the shaft is constant and the mass of the flywheel is 180 kg with radius of gyration of 35 cm. HW9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY