
Concept explainers
A piston–cylinder device contains 6 kg of H2 and 21 kg of N2 at 160 K and 5 MPa. Heat is now transferred to the device, and the mixture expands at constant pressure until the temperature rises to 200 K. Determine the heat transfer during this process by treating the mixture (a) as an ideal gas and (b) as a nonideal gas and using Amagat’s law.
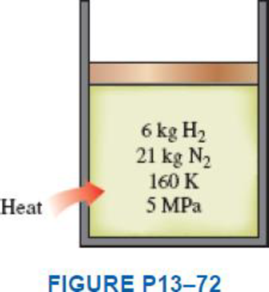
a)
The heat transfer during the process by treating as an ideal gas.
Answer to Problem 72P
The heat transfer during the process as an ideal gas is
Explanation of Solution
Write a closed system energy balance for the gas mixture.
Here, input energy is
Write the expression to obtain the mole number of
Here, molar mass of
Write the expression to obtain the mole number of
Conclusion:
Refer Table A-1, “Molar mass, gas constant, and critical point properties”, obtain the molar masses of
Substitute
Substitute
From the Table of ideal gas for
Substitute
Thus, the heat transfer during the process as an ideal gas is
b)
The heat transfer during the process by treating as non-ideal gas.
Answer to Problem 72P
The heat transfer during the process by treating as non-ideal gas is
Explanation of Solution
Write the expression to obtain the initial reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial and final reduced pressure of
Here, critical temperature of
Write the expression to obtain the final reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial and final reduced pressure of
Here, critical temperature of
Write the expression to obtain the final reduced temperature of
Here, critical temperature of
Consider hydrogen as ideal gas
Write the expression for molar enthalpy difference of hydrogen
Write the expression for molar enthalpy difference of nitrogen.
Conclusion:
Substitute 160 K for
Substitute 5 MPa for
Substitute 200 K for
Refer Figure A-30, “Generalized entropy departure chart”, obtain the value of
Substitute 160 K for
Substitute 5 MPa for
Substitute 200 K for
Refer Figure A-30, “Generalized entropy departure chart”, obtain the value of
Substitute
Substitute
Substitute
Thus, the heat transfer during the process by treating as non-ideal gas is
Want to see more full solutions like this?
Chapter 13 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
- 3.) 15.40 – Collar B moves up at constant velocity vB = 1.5 m/s. Rod AB has length = 1.2 m. The incline is at angle = 25°. Compute an expression for the angular velocity of rod AB, ė and the velocity of end A of the rod (✓✓) as a function of v₂,1,0,0. Then compute numerical answers for ȧ & y_ with 0 = 50°.arrow_forward2.) 15.12 The assembly shown consists of the straight rod ABC which passes through and is welded to the grectangular plate DEFH. The assembly rotates about the axis AC with a constant angular velocity of 9 rad/s. Knowing that the motion when viewed from C is counterclockwise, determine the velocity and acceleration of corner F.arrow_forward500 Q3: The attachment shown in Fig.3 is made of 1040 HR. The static force is 30 kN. Specify the weldment (give the pattern, electrode number, type of weld, length of weld, and leg size). Fig. 3 All dimension in mm 30 kN 100 (10 Marks)arrow_forward
- (read image) (answer given)arrow_forwardA cylinder and a disk are used as pulleys, as shown in the figure. Using the data given in the figure, if a body of mass m = 3 kg is released from rest after falling a height h 1.5 m, find: a) The velocity of the body. b) The angular velocity of the disk. c) The number of revolutions the cylinder has made. T₁ F Rd = 0.2 m md = 2 kg T T₂1 Rc = 0.4 m mc = 5 kg ☐ m = 3 kgarrow_forward(read image) (answer given)arrow_forward
- 11-5. Compute all the dimensional changes for the steel bar when subjected to the loads shown. The proportional limit of the steel is 230 MPa. 265 kN 100 mm 600 kN 25 mm thickness X Z 600 kN 450 mm E=207×103 MPa; μ= 0.25 265 kNarrow_forwardT₁ F Rd = 0.2 m md = 2 kg T₂ Tz1 Rc = 0.4 m mc = 5 kg m = 3 kgarrow_forward2. Find a basis of solutions by the Frobenius method. Try to identify the series as expansions of known functions. (x + 2)²y" + (x + 2)y' - y = 0 ; Hint: Let: z = x+2arrow_forward
- 1. Find a power series solution in powers of x. y" - y' + x²y = 0arrow_forward3. Find a basis of solutions by the Frobenius method. Try to identify the series as expansions of known functions. 8x2y" +10xy' + (x 1)y = 0 -arrow_forwardHello I was going over the solution for this probem and I'm a bit confused on the last part. Can you please explain to me 1^4 was used for the Co of the tubular cross section? Thank you!arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





