
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 87IL
A solution of benzoic acid in benzene has a freezing point of 3.1 °C and a boiling point of 82.6 °C. (The freezing point of pure benzene is 5.50 °C, and its boiling point is 80.1 °C) The structure of benzoic acid is
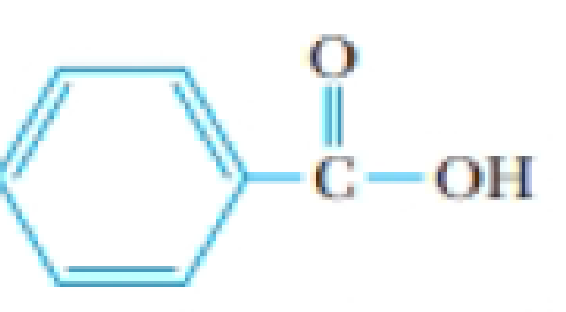
Benzoic acid, C6H5CO2H
What can you conclude about the state of the benzoic acid molecules at the two different temperatures? Recall the discussion of hydrogen bonding in Section 11.3.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total
stereoisomers are there? Name the sugar you drew.
Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total
stereoisomers are there? Draw the enantiomer.
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Which of the following molecules are NOT typical carbohydrates? For the molecules that are
carbohydrates, label them as an aldose or ketose.
HO
Он
ОН ОН
Он
ОН
но
ΤΗ
HO
ОН
HO
eve
Он он
ОН
ОН
ОН
If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units
are present?
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Ch. 13.1 - (a) If you dissolve 10.0 g (about one heaping...Ch. 13.1 - You dissolve 1.0 mol of urea (H2NCONH2) in 270 g...Ch. 13.1 - 2. The concentration of acetic acid, CH3CO2H, in a...Ch. 13.2 - Use the data in Table 13.1 to calculate the...Ch. 13.2 - Given the enthalpy of formation data below,...Ch. 13.3 - Prob. 1CYUCh. 13.3 - Prob. 1RCCh. 13.3 - If the headspace of a soda is 25 mL and the...Ch. 13.3 - Prob. 2QCh. 13.3 - Prob. 3Q
Ch. 13.3 - Prob. 4QCh. 13.4 - Assume you dissolve 10.0 g of sucrose (C12H22O11)...Ch. 13.4 - What quantity of ethylene glycol, HOCH2CH2OH, must...Ch. 13.4 - In the northern United States, summer cottages are...Ch. 13.4 - Bradykinin is a small peptide (9 amino acids; 1060...Ch. 13.4 - An aluminum-containing compound has the empirical...Ch. 13.4 - A 1.40-g sample of polyethylene, a common plastic,...Ch. 13.4 - Calculate the freezing point of 525 g of water...Ch. 13.4 - 1. Vapor pressure: Arrange the following aqueous...Ch. 13.4 - Prob. 2RCCh. 13.4 - Samples of each of the substances listed below are...Ch. 13.4 - Motor mass: Erythritol is a compound that occurs...Ch. 13.5 - Prob. 1RCCh. 13.5 - The blue line on the diagram illustrates the...Ch. 13.5 - How many theoretical plates are required to...Ch. 13.5 - Prob. 3QCh. 13.5 - The vapor pressure of pure heptane is 361.5 mm Hg...Ch. 13 - You dissolve 2.56 g of succinic acid, C2H4(CO2H)2,...Ch. 13 - You dissolve 45.0 g of camphor, C10H16O, in 425 mL...Ch. 13 - Prob. 3PSCh. 13 - Prob. 4PSCh. 13 - Prob. 5PSCh. 13 - Prob. 6PSCh. 13 - Prob. 7PSCh. 13 - Prob. 8PSCh. 13 - Hydrochloric acid is sold as a concentrated...Ch. 13 - Concentrated sulfuric acid has a density of 1.84...Ch. 13 - The average lithium ion concentration in seawater...Ch. 13 - Silver ion has an average concentration of 28 ppb...Ch. 13 - Which pairs of liquids will be miscible? (a) H2O...Ch. 13 - Acetone, CH3COCH3, is quite soluble in water....Ch. 13 - Prob. 15PSCh. 13 - Use the following data to calculate the enthalpy...Ch. 13 - You make a saturated solution of NaCl at 25 C. No...Ch. 13 - Some lithium chloride, LiCl, is dissolved in 100...Ch. 13 - Prob. 19PSCh. 13 - The Henrys law constant for O2 in water at 25 is...Ch. 13 - An unopened soda can has an aqueous CO2...Ch. 13 - Hydrogen gas has a Henrys law constant of 7.8 104...Ch. 13 - A sealed flask contains water and oxygen gas at 25...Ch. 13 - Butane, C4H10, has been suggested as the...Ch. 13 - A 35.0-g sample of ethylene glycol, HOCH2CH2OH, is...Ch. 13 - Urea, (NH2)2CO, which is widely used in...Ch. 13 - Pure ethylene glycol, HOCH2CH2OH, is added 2.00 kg...Ch. 13 - Pure iodine (105 g) is dissolved in 325 g of CCl4...Ch. 13 - Prob. 29PSCh. 13 - What is the boiling point of a solution composed...Ch. 13 - Prob. 31PSCh. 13 - Prob. 32PSCh. 13 - Prob. 33PSCh. 13 - Some ethylene glycol, HOCH2CH2OH, is added to your...Ch. 13 - You dissolve 15.0 g of sucrose, C12H22O11, in a...Ch. 13 - A typical bottle of wine consists of an 11%...Ch. 13 - Prob. 37PSCh. 13 - Estimate the osmotic pressure of human blood at 37...Ch. 13 - An aqueous solution containing 1.00 g of bovine...Ch. 13 - Calculate the osmotic pressure of a 0.0120 M...Ch. 13 - You add 0.255 g of an orange, crystalline compound...Ch. 13 - Butylated hydroxyanisole (BHA) is used in...Ch. 13 - Benzyl acetate is one of the active components of...Ch. 13 - Anthracene, a hydrocarbon obtained from coal, has...Ch. 13 - An aqueous solution contains 0.180 g of an...Ch. 13 - Aluminon, an organic compound, is used as a...Ch. 13 - Prob. 47PSCh. 13 - To make homemade ice cream, you cool the milk and...Ch. 13 - List the following aqueous solutions in order of...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - When solutions of BaCl2 and Na2SO4 are mixed, the...Ch. 13 - The dispersed phase of a certain colloidal...Ch. 13 - Phenylcarbinol is used in nasal sprays as a...Ch. 13 - (a) Which aqueous solution is expected to have the...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - Prob. 56GQCh. 13 - Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a...Ch. 13 - A 10.7 m solution of NaOH has a density of 1.33...Ch. 13 - Concentrated aqueous ammonia has a molarity of...Ch. 13 - Prob. 60GQCh. 13 - If you want a solution that is 0.100 m in ions,...Ch. 13 - Consider the following aqueous solutions: (i) 0.20...Ch. 13 - (a) Which solution is expected to have the higher...Ch. 13 - The solubility of NaCl in water at 100 C is 39.1...Ch. 13 - Instead of using NaCl to melt the ice on your...Ch. 13 - The smell of ripe raspberries is due to...Ch. 13 - Hexachlorophene has been used in germicidal soap....Ch. 13 - The solubility of ammonium formate, NH4CHO2, in...Ch. 13 - How much N2 can dissolve in water at 25 C if the...Ch. 13 - Cigars are best stored in a humidor at 18 C and...Ch. 13 - An aqueous solution containing 10.0 g of starch...Ch. 13 - Prob. 72GQCh. 13 - Calculate the enthalpies of solution for Li2SO4...Ch. 13 - Water at 25 C has a density of 0.997 g/cm3....Ch. 13 - If a volatile solute is added to a volatile...Ch. 13 - A solution is made by adding 50.0 mL of ethanol...Ch. 13 - A 2.0% (by mass) aqueous solution of novocainium...Ch. 13 - A solution is 4.00% (by mass) maltose and 96.00%...Ch. 13 - The following table lists the concentrations of...Ch. 13 - A tree is 10.0 m tall. (a) What must be the total...Ch. 13 - Prob. 81GQCh. 13 - A compound is known to be a potassium halide, KX....Ch. 13 - Prob. 85GQCh. 13 - If one is very careful, it is possible to float a...Ch. 13 - A solution of benzoic acid in benzene has a...Ch. 13 - You dissolve 5.0 mg of iodine, I2, in 25 mL of...Ch. 13 - Prob. 89ILCh. 13 - In a police forensics lab, you examine a package...Ch. 13 - An organic compound contains carbon (71.17%),...Ch. 13 - Prob. 92ILCh. 13 - When sails of Mg2+, Ca2+, and Be2+ are placed in...Ch. 13 - Explain why a cucumber shrivels up when it is...Ch. 13 - Prob. 95SCQCh. 13 - A 100.-gram sample of sodium chloride (NaCl) is...Ch. 13 - Prob. 97SCQCh. 13 - Prob. 98SCQCh. 13 - Starch contains CC, CH, CO, and OH bonds....Ch. 13 - Prob. 100SCQCh. 13 - You have two aqueous solutions separated by a...Ch. 13 - Prob. 102SCQCh. 13 - Sodium chloride (NaCl) is commonly used to melt...Ch. 13 - Prob. 105SCQCh. 13 - Prob. 106SCQCh. 13 - Prob. 107SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Give the IUPAC name for each compound.
Organic Chemistry
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward
- 1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forward
- What is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward
- 3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Intermolecular Forces and Boiling Points; Author: Professor Dave Explains;https://www.youtube.com/watch?v=08kGgrqaZXA;License: Standard YouTube License, CC-BY